Preparation method for curcumin derivative
A curcumin derivative and a technology for curcumin are applied in the field of preparation of curcumin derivatives, can solve the problems of unsustainable drug effect, low selectivity, low bioavailability, etc., achieve improved pharmacological or biological activity, and simple process , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of curcumin transformation product
[0038] (1) Slant culture: inoculate Rhizopus oligosporus varietal ZJPH1308 to the slant medium, and cultivate for 4 to 5 days at 30°C to obtain the slant strain; the final concentration of the slant medium consists of: potato 200g / L, Glucose 20g / L, agar powder 20g / L, pH natural, solvent is water, sterilized at 121°C for 20 minutes, cooled after sterilization, and made into a slope;
[0039] (2) Seed culture: pick a ring of thalli from the slant of step (1) and inoculate it into a 250mL shake flask with 50mL of seed culture medium, cultivate it at 30°C and 200rpm for 22h, and prepare a seed solution; the seed culture The base final concentration is composed of: glucose 25g / L, peptone 27.5g / L, (NH 4 ) 2 SO 4 3g / L, KH 2 PO 4 1g / L, MgSO 4 ·7H 2 O 1.3g / L, pH 6.0, solvent is water, sterilized at 121°C for 20 minutes;
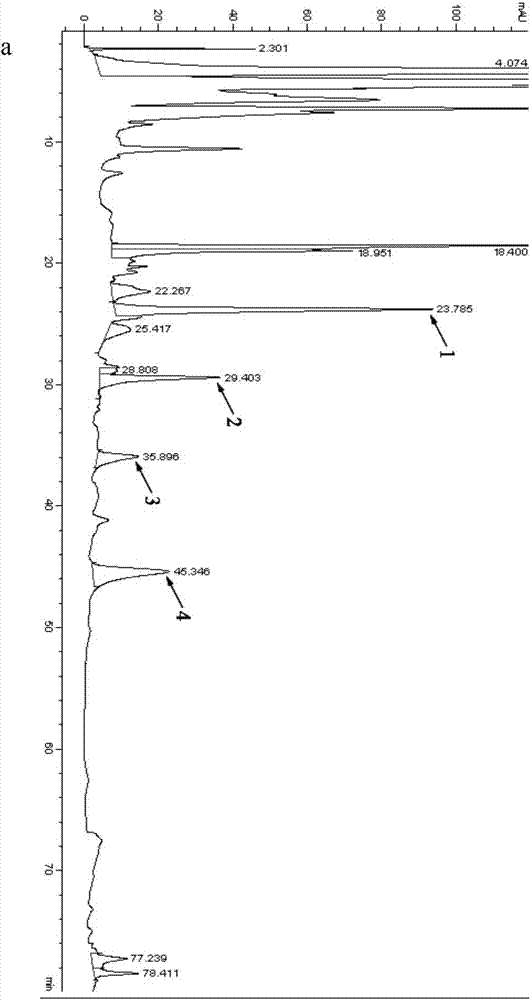
[0040] (3) Initial fermentation culture for the preparation of curcumin derivative...
Embodiment 2-8
[0066] The impact of embodiment 2-8 carbon source type on conversion product 4 yield
[0067] Utilize Rhizopus oligosporus strain ZJPH1308, based on the initial fermentation medium, replace glucose with maltose, glycerol, soluble starch, sucrose, dextrin, and lactose at a concentration of 25g / L as the sole carbon source to change the initial fermentation The culture medium is transformed according to the method of step (4) of Example 1. After the transformation is completed, the transformation liquid is centrifuged to remove the bacteria to obtain a supernatant, which is extracted three times with ethyl acetate, and the extracts are combined and stored at 50 ° C. Concentrate under reduced pressure to dryness, take the concentrate and dissolve it in chromatographic methanol for detection. The detection method is the same as that of step (5) in Example 1, and the yield of product 4 (t=45.3min) is calculated according to the area normalization method. The results are shown in Tab...
Embodiment 9-13
[0071] The influence of embodiment 9-13 dextrin concentration on conversion product 4 yield
[0072] On the basis of Example 7, prepare the initial fermentation medium whose dextrin concentration is 10g / L, 20g / L, 25g / L, 30g / L, and 40g / L respectively, change the initial fermentation medium, and follow the steps of Example 1 The method of (4) is transformed. After the transformation is completed, the transformation liquid is centrifuged to remove the bacterial cells to obtain a supernatant, which is extracted three times with ethyl acetate, and the extracts are combined, concentrated to dryness at 50 ° C under reduced pressure, and the concentrated The substance is detected after being dissolved in chromatographic methanol, and the detection method is the same as that of Example 1 step (5), and the yield of product 4 (t=45.3min) is calculated according to the area normalization method. The results are shown in Table 4.
[0073] The influence of table 4 different dextrin concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com