Spinosad hapten and preparation method and application thereof
A spinosyn and hapten technology, applied in the field of hapten and its preparation, can solve the problems of many processing steps and low recovery rate, and achieve the effects of low detection cost, good affinity and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
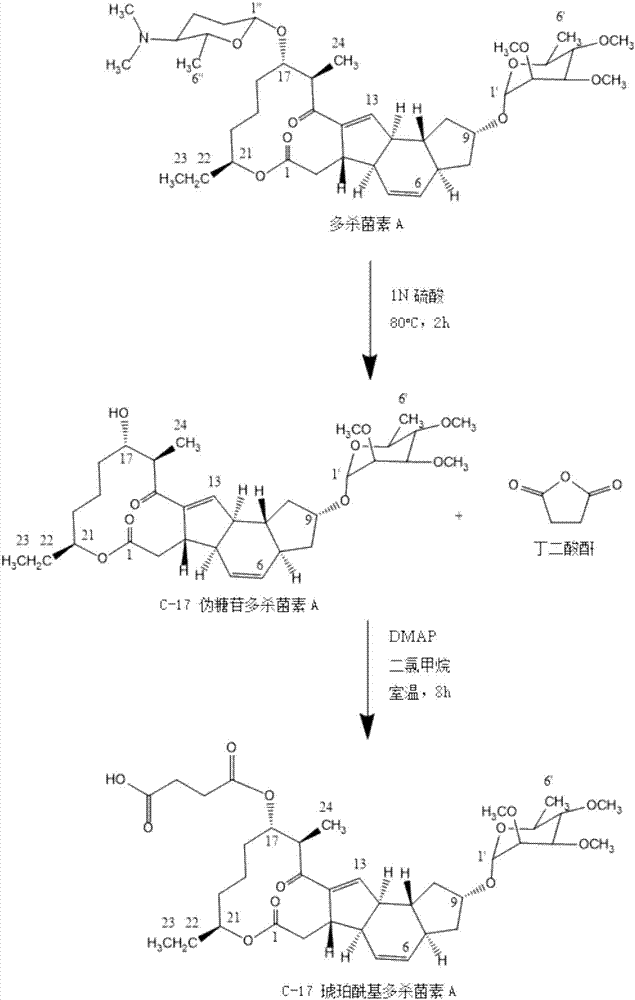
[0024] Example 1: Synthesis of spinosad haptens
[0025] step 1:
[0026] Weigh 1g, that is, 1.37mmol spinosad in a 50ml eggplant-shaped flask, dissolve it in 30ml 1N sulfuric acid, heat to 80°C, stir the reaction with a magnetic force, and monitor the completion of the reaction on a TLC board (developing agent is ethyl acetate:petroleum ether=7: 3); wash the precipitate with 50ml of dilute sulfuric acid, add 30ml of dichloromethane to dissolve, transfer to a separatory funnel, wash twice with saturated sodium chloride solution, and collect the organic phase; after drying with anhydrous sodium sulfate, filter and spin to dry the solvent, The hapten intermediate was purified by 200-300 mesh silica gel column chromatography, the eluent was ethyl acetate:petroleum ether, the volume ratio was 7:3, and the yield was 71.4%.
[0027] Step 2:
[0028] Weigh 600mg of the hapten intermediate into a brown bottle, add 750mg of succinic anhydride and 122.2mg of 4-dimethylaminopyridine, d...
Embodiment 2
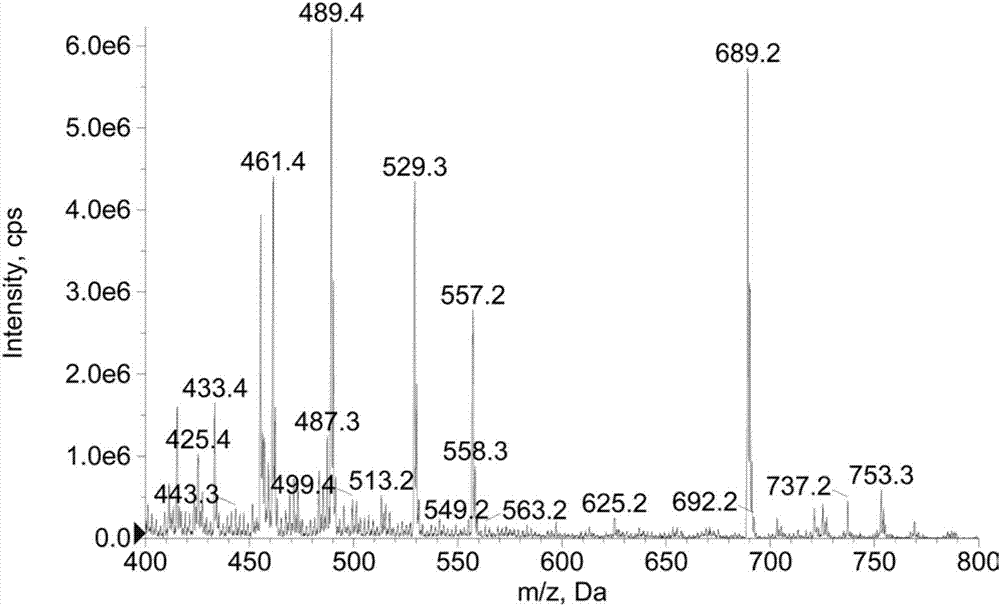
[0029] Example 2: Identification of spinosad haptens
[0030] The product of the above-mentioned Example 1 was measured by H NMR and C NMR, and the NMR data were as follows, indicating that the hapten was successfully synthesized:
[0031] 1H NMR (500MHz, CD3OD) δ7.09(s, 1H), 5.93(d, J=9.8Hz, 1H), 5.86(dt, J=9.8, 2.8Hz, 1H), 5.03–4.95(m, 1H) ,4.87(d,J=1.7Hz,2H),4.70–4.61(m,1H),4.34(dd,J=12.7,7.1Hz,1H),3.60–3.52(m,3H),3.52(s,3H ),3.48–3.44(m,9H),3.43(t,J=3.3Hz,1H),3.35(s,6H),3.08(dt,J=18.8,7.2Hz,2H),2.98-2.96(m, 1H),2.91–2.83(m,1H),2.64–2.58(m,4H),2.47(dd,J=13.4,3.2Hz,1H),2.41–2.30(m,1H),2.22–2.12(m, 1H),2.04–1.93(m,1H),1.66–1.27(m,11H),1.24(d,J=6.2Hz,3H),1.09(d,J=6.7Hz,3H),0.99–0.88(m ,1H),0.83(t,J=7.5Hz,3H).
[0032] 13C NMR(126MHz,MeOD)δ203.44,175.96,173.93,173.92,150.37,145.06,130.66,129.74,97.06,83.55,82.52,78.63,77.96,77.91,76.29,69.10,61.14,59.15,57.72,51.06,49.85,47.46 ,46.72,42.87,42.55,38.54,37.44,35.01,33.58,31.11,30.27,29.84,29.28,22.34,18.13,16.45,9.62.
Embodiment 3
[0033] Example 3: Spinosyn antigen
[0034] Add 0.6ml of anhydrous DMF to 0.09mmol of the succinyl spinosyn hapten, mix well, add 0.12mmol of DCC and 0.12mmol of NHS to activate the carboxyl group on the hapten, stir overnight, then centrifuge To remove precipitation, divide the solution into three parts on average, and add them dropwise to different carrier protein solutions. The carrier protein is BSA, OVA, KLH, etc., and the buffer for dissolving the protein is carbonate buffer or boron Salt buffer solution, the amount of protein used is calculated according to the molar ratio of hapten to protein 15-40:1. After the coupling reaction between the carboxyl-activated hapten and the amino group on the protein molecule overnight, the reaction solution was transferred to a semi-permeable membrane, and dialyzed at 4°C for 3-5 days using 10 mM PBS buffer solution of pH 7.2 to obtain a coating Originally, the spinosad hapten was successfully coupled to the carrier protein after ult...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com