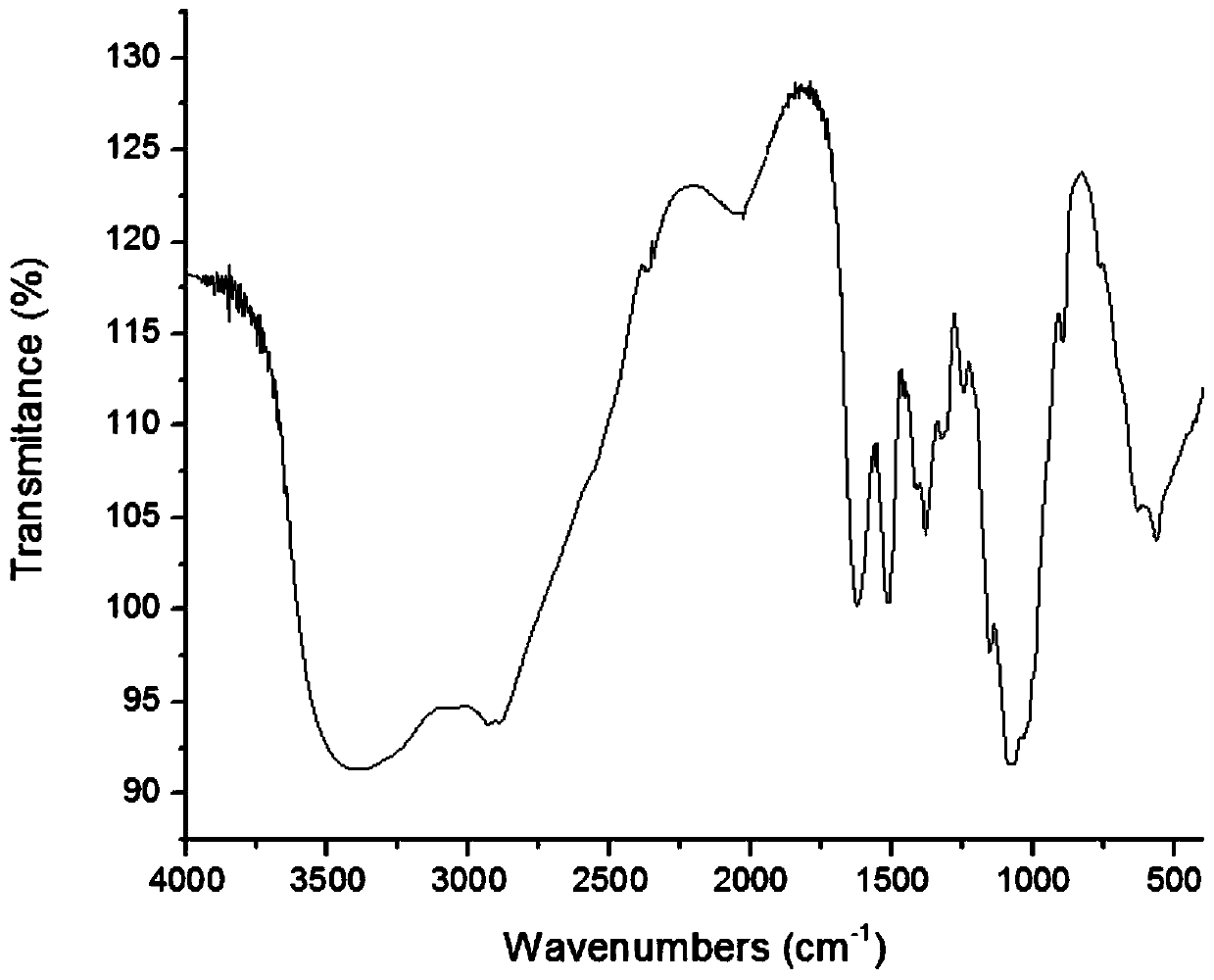
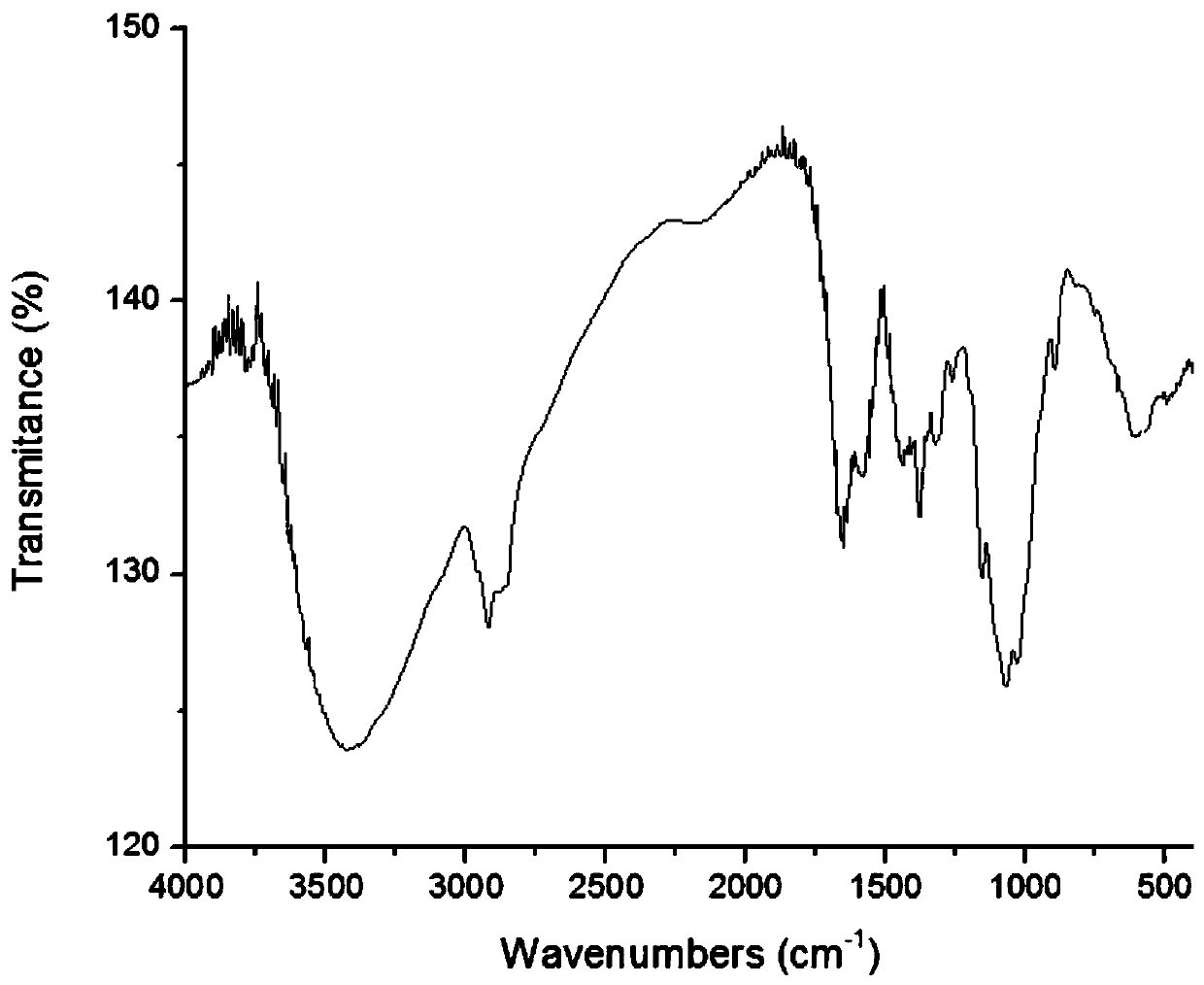
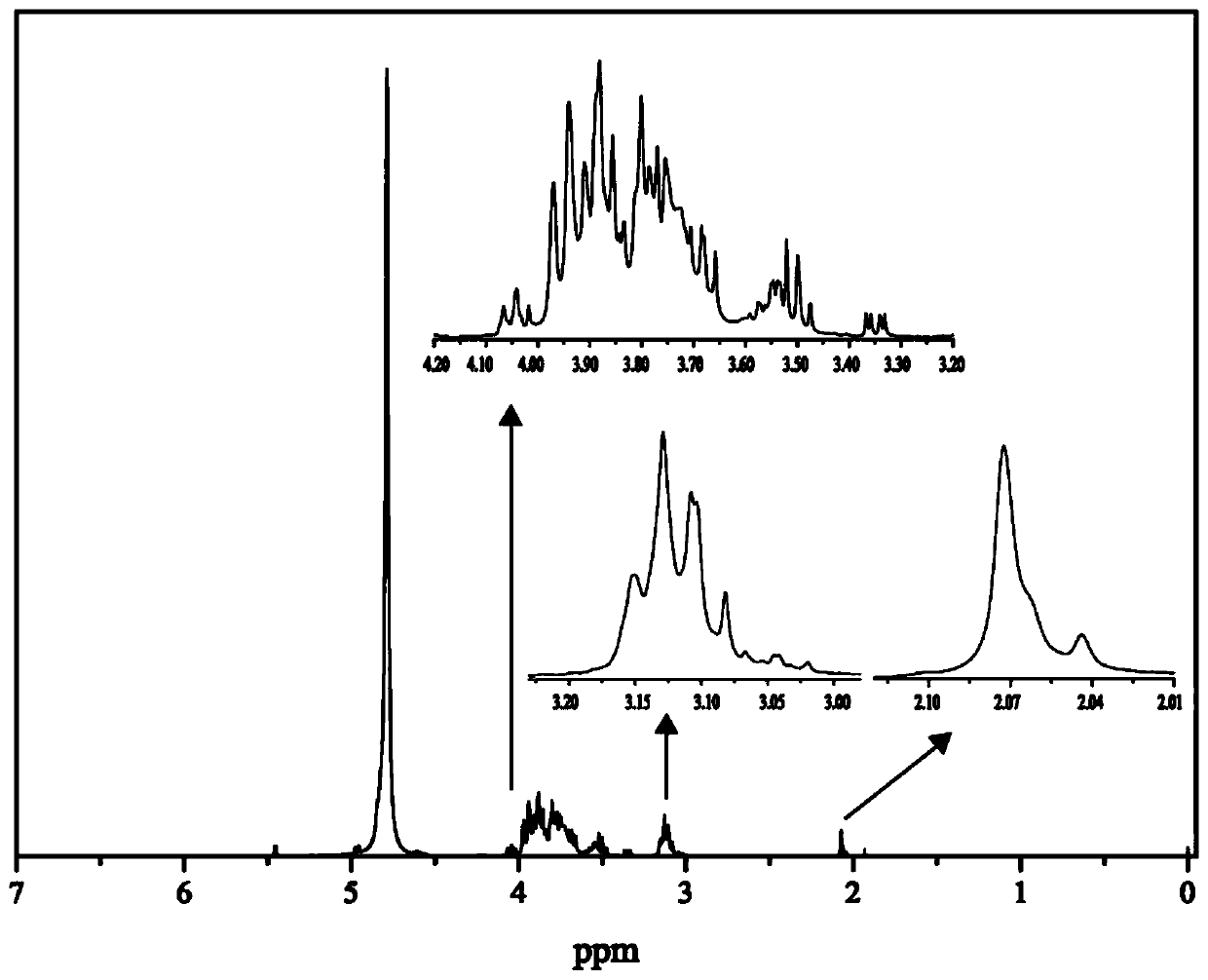
A kind of chitosan oligosaccharide-o-geraniol derivative and its preparation method and application
A technology of chitosan oligosaccharide and geraniol is applied in the field of chitosan oligosaccharide-O-geraniol derivatives and the preparation thereof, which can solve the problems of low antibacterial activity and uncommon application, and achieves low cost and good water solubility. , the effect of stable nature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation method of oligochitosan-O-geraniol derivative comprises the following steps:
[0040] 1. Preparation of Geranyl Bromide
[0041] Take geraniol in a three-necked flask, add anhydrous ether and catalyst. Cool in an ice-salt bath to -5°C, add phosphorus tribromide dropwise, and the dropwise addition is completed in about 15 minutes. After continuing to react for 15 minutes, pour the solution into a separatory funnel, wash the precipitate with ether and put it into the separatory funnel, wash it with 5% sodium bicarbonate solution until neutral, wash it with water, wash it with saturated saline, and wash it with anhydrous magnesium sulfate. Dry, filter, and precipitate under reduced pressure to obtain geranyl bromide.
[0042] 2. Preparation of Oligochitosan-O-geraniol Derivatives
[0043] Chitooligosaccharides with a molecular weight of 1000 Da and a degree of polymerization n of 4 to 7 were selected, and the chitooligosaccharide Schiff base was prepared...
Embodiment 2
[0053] The preparation method of oligochitosan-O-geraniol derivative comprises the following steps:
[0054] 1. Preparation of Geranyl Bromide
[0055] Take geraniol in a three-necked flask, add anhydrous ether and catalyst. Cool in an ice-salt bath to -5°C, add phosphorus tribromide dropwise, and the dropwise addition is completed in about 15 minutes. After continuing to react for 15 minutes, pour the solution into a separatory funnel, wash the precipitate with ether and put it into the separatory funnel, wash it with 5% sodium bicarbonate solution until neutral, wash it with water, wash it with saturated saline, and wash it with anhydrous magnesium sulfate. Dry, filter, and precipitate under reduced pressure to obtain geranyl bromide.
[0056] 2. Preparation of Oligochitosan-O-geraniol Derivatives
[0057] Oligochitosan with a molecular weight of 15000 Da and a degree of polymerization n of 95-100 was selected, and oligochitosan Schiff base was prepared by protecting the ...
Embodiment 3
[0062] The preparation method of oligochitosan-O-geraniol derivative comprises the following steps:
[0063] 1. Preparation of Geranyl Bromide
[0064] Take geraniol in a three-necked flask, add anhydrous ether and catalyst. Cool in an ice-salt bath to -5°C, add phosphorus tribromide dropwise, and the dropwise addition is completed in about 15 minutes. After continuing to react for 15 minutes, pour the solution into a separatory funnel, wash the precipitate with ether and put it into the separatory funnel, wash it with 5% sodium bicarbonate solution until neutral, wash it with water, wash it with saturated saline, and wash it with anhydrous magnesium sulfate. Dry, filter, and precipitate under reduced pressure to obtain geranyl bromide.
[0065] 2. Preparation of Oligochitosan-O-geraniol Derivatives
[0066] Chitooligosaccharides with a molecular weight of 5000 Da and a degree of polymerization n of 30 to 35 were selected, and the chitooligosaccharide Schiff base was prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com