sH2a monoclonal antibody hybridoma and monoclonal antibody and application thereof
A monoclonal antibody and hybridoma cell technology, which is applied in the field of protein detection, can solve the problems of insufficient sensitivity, specificity, insufficient antibody efficiency, and low titer, and achieve shortened reaction time, sufficient and rapid response, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1, expression, purification and identification of sH2a recombinant protein
[0069] 1. Prokaryotic protein expression
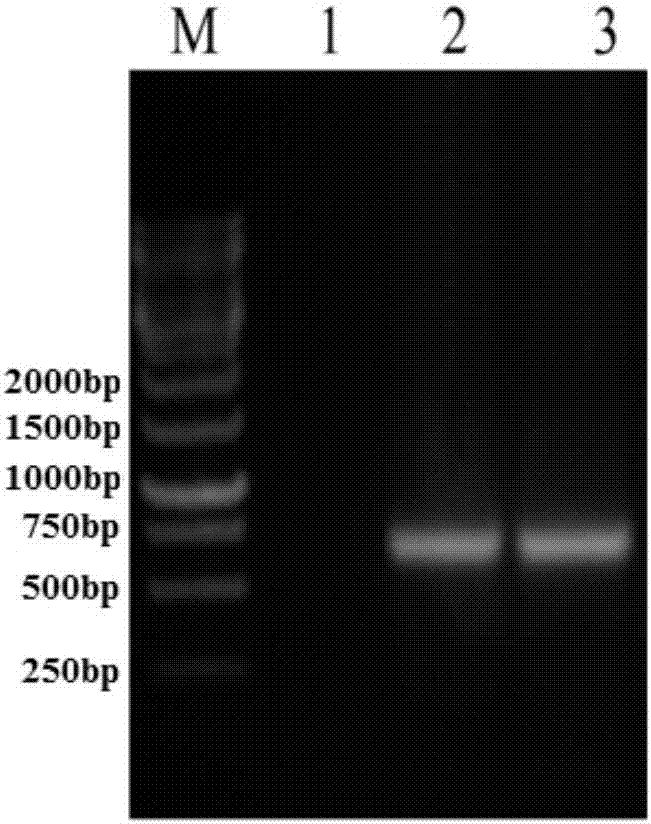
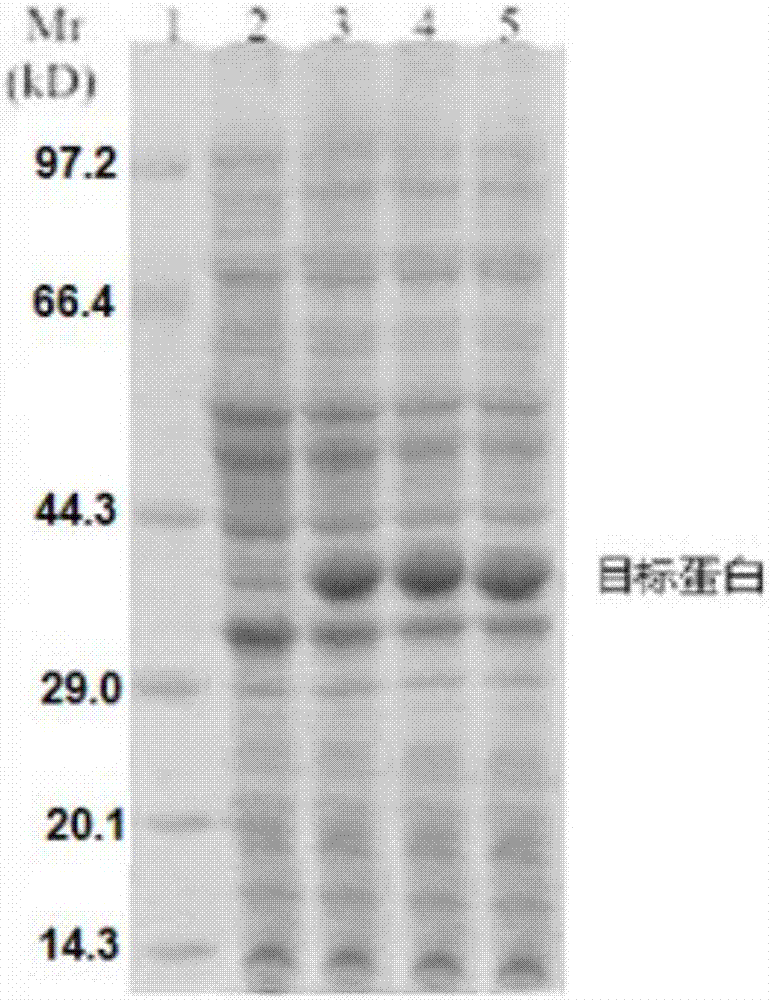
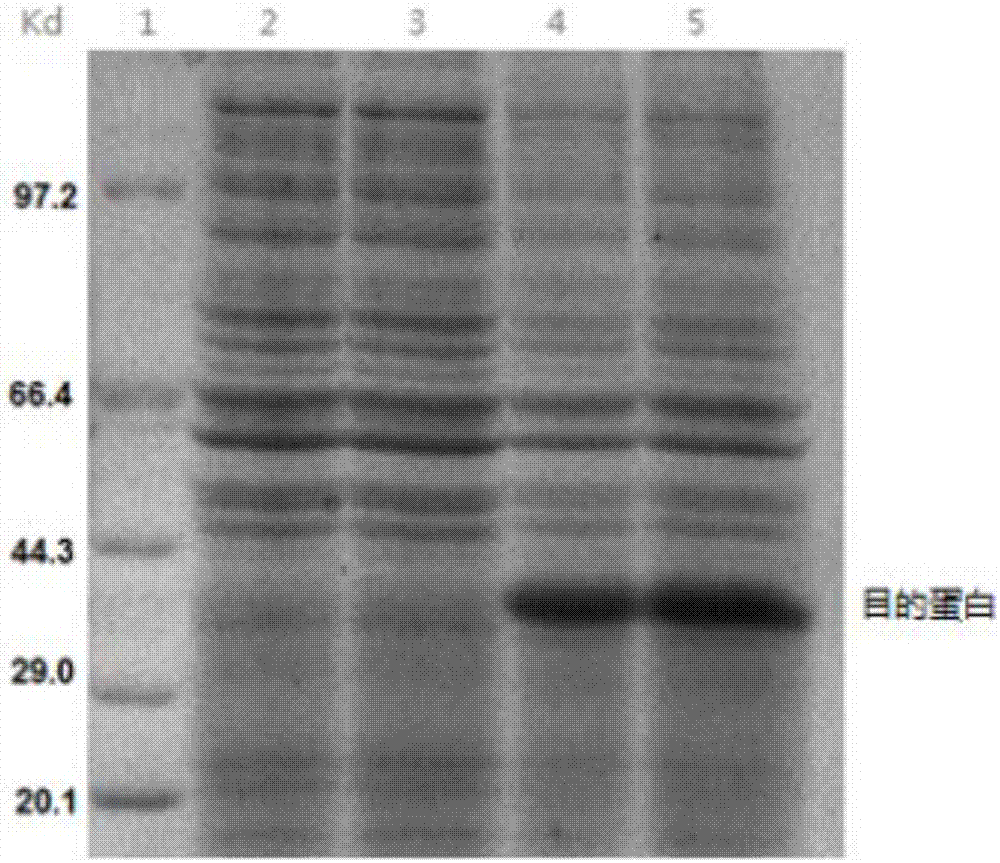
[0070] Using liver cancer tissue cDNA as a template, the upstream primer 5'-GGATCCGCACAGCTGCAAGCCGAGCTGCG-3' (SEQ ID No.1) and the downstream primer 5'-GAATTCTCAGGCCACCTCGCCGGTGGCA-3' (SEQ ID No.2) were used as primers to amplify the sH2a gene fragment by PCR ( figure 1 ), after gel cutting, recovery and purification, it was connected to the cloning vector pCR2.1 and sequenced for identification, and then the sH2a gene fragment with the correct sequence identification was cloned into the prokaryotic expression vector pET30a(+), and the restriction sites were BamH I and EcoR I , to obtain the recombinant plasmid pET30a-sH2a. The recombinant plasmid pET30a-sH2a was transformed into Escherichia coli BL21(DE3), and the successfully transformed clones were picked, cultured at 37°C until the OD600nm value was about 0.6, and 1mM IPTG was added to...
Embodiment 2
[0076] Embodiment 2, preparation of anti-sH2a monoclonal antibody
[0077] 1. Antigen-immunized mice
[0078] Two 6-week-old BALB / c female mice were taken and immunized according to the following steps:
[0079] First immunization: mix the sH2a prokaryotic recombinant protein solution with Freund’s complete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml (30 μg / mouse of sH2a recombinant protein) subcutaneously at two points on the back of the mouse;
[0080] The second immunization: after an interval of 14 days, mix the sH2a recombinant protein solution with Freund's incomplete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml (sH2a recombinant Protein 50μg / piece);
[0081] The third immunization: after an interval of 14 days, mix the sH2a recombinant protein solution with Freund’s incomplete adjuvant at a volume ratio of 1:1, fully emulsify, and inject 0.1ml subcutaneously at the back of the shoulder and abdomen on both sides of the mou...
Embodiment 3
[0093] Example 3, paired screening of anti-sH2a monoclonal antibodies
[0094] 1. Antibody Pair Screening
[0095] The double-antibody sandwich method was used for pairwise paired experiments. The above-mentioned five anti-sH2a monoclonal antibodies with higher titers were respectively coated in solid-phase microwell plates, the antibody concentration was 5ug / ml, 100ul / well, blocked with 2% BSA phosphate buffer for 2h, and the sH2a prokaryotic The recombinant protein was diluted to 500ng / ml, and continued to be diluted to 6 concentrations. The diluted solution was used as a blank control, and reacted at 37°C for 1 hour, and the other 4 anti-sH2a strains labeled with horseradish peroxidase (HRP) were added except itself. Monoclonal antibody (1:10000), detect the absorbance value (wavelength 450nm). The results show that the pairing of anti-sH2a monoclonal antibody 1F2D2 and 5A9B8 can detect a significant concentration gradient of the protein, which can be used to establish a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com