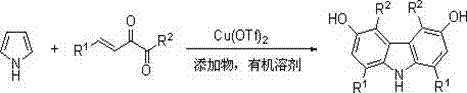
Preparation method of hydroxyl-containing carbazole compound with symmetrical structure
A technology with symmetrical structures and compounds, which is applied in the field of carbazole compound preparation, can solve problems such as the inability to synthesize carbazole compounds quickly and efficiently, expensive and difficult to obtain precious metal catalysts, and limitations of substrate universality, etc., and achieves a fast synthesis rate , easy operation, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
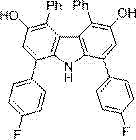
[0025] Synthesis of 1, 4, 5, 8-tetraphenyl-3, 6-dihydroxy-9H-carbazole
[0026] Add 34 mg (ie 0.5 mmol) of pyrrole, 354 mg (ie 1.5 mmol) of 1,4-diphenyl-3-butene-1,2-dione, 36 mg (ie 0.1 mmol) into a 25 mL glass reaction vial ) Trifluoromethanesulfonic acid ketone, then add 4 mL 1,4-dioxane, at 25 o C under stirring for 2 hours, adding 10 mg (i.e. 0.05 mmol) p-toluenesulfonic acid monohydrate, at 70 o Stir at C for 3 hours, concentrate after reaction, use petroleum ether: ethyl acetate volume ratio of 10:1 as eluent, and purify by silica gel column chromatography to obtain 1, 4, 5, 8-tetraphenyl-3 , 6-dihydroxy-9H-carbazole, its structure is shown in the following formula:
[0027]
[0028] This compound is yellow solid, and productive rate is 52%, and its nuclear magnetic data is as follows:
[0029] 1 H NMR (500 MHz, CDCl 3 ) δ 4.75 (s, 1H), 6.85 - 6.70 (dd, J 1 = 7.0 Hz, J 2 =1.5 Hz, 4H), 7.03 - 7.11 (s, 6 H), 7.13 (s, 2H), 7.40 - 7.43 (t, J = 7.5 Hz,2H), 7....
Embodiment 2
[0031] Synthesis of 1, 8-bis(4-fluorophenyl)-4, 5-diphenyl-9H-carbazole
[0032] Add 34 mg (ie 0.5 mmol) of pyrrole and 381 mg (ie 1.5 mmol) of 1-(4-fluorophenyl)-4-phenyl-3-butene-1,2-dione into a 25mL glass reaction vial , 36 mg (ie 0.1 mmol) trifluoromethanesulfonic acid ketone, then add 4mL 1,4-dioxane, at 25 o C under stirring for 1 hour, adding 10 mg (ie 0.05 mmol) p-toluenesulfonic acid monohydrate, at 70 o Stirring at C for 3 hours, concentrated after reaction, using petroleum ether:ethyl acetate volume ratio of 10:1 as eluent, purified by silica gel column chromatography to obtain 1,8-bis(4-fluorophenyl)- 4, 5-diphenyl-9H-carbazole, its structure is shown in the following formula:
[0033]
[0034] This compound is yellow solid, and productive rate is 46%, and its NMR data is as follows:
[0035] 1 H NMR (500 MHz, CDCl 3 ) δ 4.77 (s, 2H), 6.82 - 6.84 (dd, J 1 = 7.5Hz, J 2 =1.5 Hz, 4H), 7.17 - 7.12 (m, 8 H), 7.50 - 7.52 (d, J = 8.5 Hz, 4H), 7.61 -7.63 (...
Embodiment 3
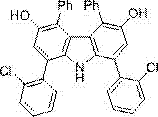
[0037] Synthesis of 1, 8-bis(2-chlorophenyl)-4, 5-diphenyl-9H-carbazole
[0038] Add 34 mg (ie 0.5 mmol) of pyrrole and 407 mg (ie 1.5 mmol) of 1-(2-chlorophenyl)-4-phenyl-3-butene-1, 2-dione into a 25 mL glass reaction vial , 36 mg (ie 0.1 mmol) trifluoromethanesulfonic acid ketone, then add 5mL 1,4-dioxane, at 25 o C under stirring for 1 hour, adding 10 mg (ie 0.05 mmol) p-toluenesulfonic acid monohydrate, at 70 o Stirring at C for 4 hours, concentrated after reaction, using petroleum ether: ethyl acetate volume ratio of 10:1 as eluent, purified by silica gel column chromatography to obtain 1, 8-bis(2-chlorophenyl)- 4, 5-diphenyl-9H-carbazole, its structure is shown in the following formula:
[0039]
[0040]This compound is yellow solid, and productive rate is 37%, and its NMR data is as follows:
[0041] 1 H NMR (500 MHz, CDCl 3 ) δ 4.75 (s, 2H), 6.85 - 6.87 (dd, J 1 = 7.5Hz, J 2 =1.5 Hz,, 4H), 7.07 - 7.10 (m, 6H), 7.13 (s, 2H) 7.23 - 7.30 (m, 4H), 7.38 -7.41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com