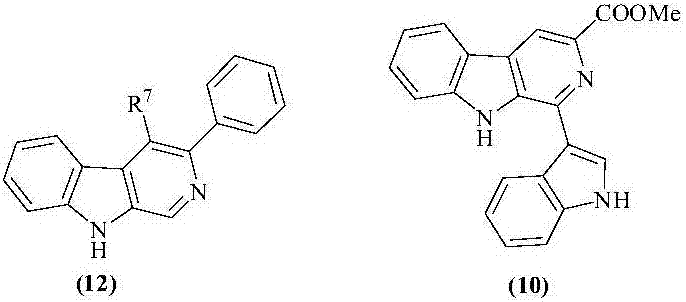
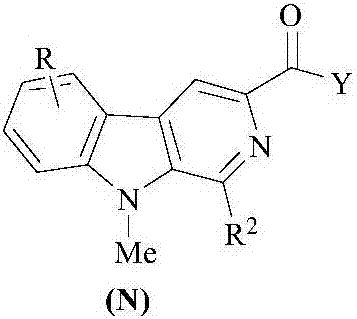
Beta-carboline derivatives, gamma-carboline derivatives as well as preparation methods and use thereof
A derivative, carboline technology, applied in the field of biosynthesis, can solve the problems of strict reaction conditions, difficult control, human and environmental hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The synthetic method of the β-carboline derivative provided by the invention specifically comprises the following steps:
[0070] Using compound (N.1) as a starting material, react with N,O-dimethylhydroxylamine hydrochloride to generate compound (N.2);
[0071] Described compound (N.2) is mixed with NaH again, CH 3 I reacts to generate compound (N.3);
[0072] The compound (N.3) undergoes a nucleophilic substitution reaction with a Grignard reagent to generate compound (N.4);
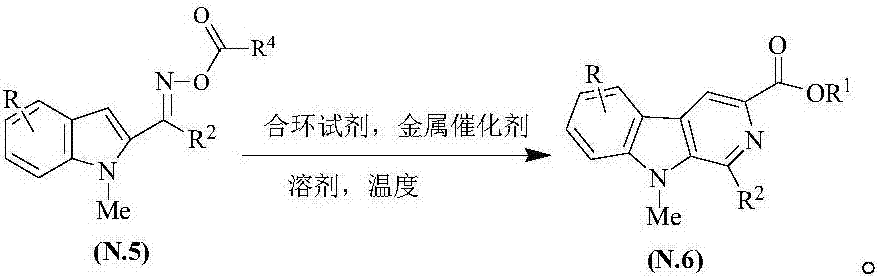
[0073] The compound (N.4) is oximated and formed into an ester to generate compound (N.5);
[0074] The compound (N.5) and the ring-closing reagent (A) or (B) are reacted at 45-150° C. for 8-36 hours in an appropriate reaction solvent under the action of a palladium reagent or a rhodium reagent as a catalyst, and then The β-carboline derivative (N.6) is obtained.
[0075] The compound (N.6) undergoes a further reaction to obtain the compound (N). The reaction formula is:
[0076]
[007...
Embodiment 1
[0085] Example 1: Synthesis of 1,9-dimethyl-9H-β-carboline-3-carboxylic acid methyl ester
[0086] S1: Dissolve 1.61g (10.0mmol) of indole-2-carboxylic acid in 80.0mL of anhydrous DCM (dichloromethane), add 1.17g (1.2eq) of N,O-dimethylhydroxylamine hydrochloride and react for 15 minutes , 2.1mL (1.2eq) DIPEA (N, N-diisopropylethylamine) was added dropwise, 2.30g (1.2eq) EDCI (carbodiimide) was added at 0°C, 0.06g (0.05eq) DMAP ( 4-dimethylaminopyridine), the reaction temperature was gradually raised to room temperature, reacted for 12 hours, and TLC (thin layer chromatography) showed that the raw materials disappeared; Dry over sodium sulfate, distill off DCM under reduced pressure to obtain 1.58 g of white solid, which is compound (1.2), Yield: 98.3%.
[0087] The structure of compound (1.2) is as follows:
[0088]
[0089] S2: Dissolve 0.80g (2.0eq) of 60% NaH in 80.0mL of dry DMF. After blowing the bottle with nitrogen, dissolve it in 20.0mL of dry DMF (N,N-dimethylfo...
Embodiment 2
[0098] Example 2: Synthesis of 9-methyl-1-phenyl-9H-β-carboline-3-carboxylic acid methyl ester:
[0099] Steps S1-S2 are the same as those in Embodiment 1, and will not be repeated here.
[0100] S3: After dissolving 1.10 g (5 mmol) of compound (2.3) {that is, compound (1.3) in Example 1} in 30 mL of anhydrous THF, under nitrogen protection, 3.8 mL (1.5 eq, 2 M n-hexane solution) phLi, the reaction temperature was gradually raised to room temperature, reacted for 1 hour, TLC showed that the raw materials disappeared; slowly added ice water at 0°C to quench the reaction, extracted 2-3 times with ethyl acetate, washed once with saturated sodium chloride solution , dried over anhydrous sodium sulfate, and evaporated under reduced pressure to obtain 0.92 g of white solid (1-methyl-1H-indol-2-yl)-phenyl-methanone, Yield: 83.6%.
[0101] S4: After dissolving 0.94g (4mmol) (1-methyl-1H-indole)-2-phenyl-methanone in 20mL absolute ethanol, add 0.81g (3.0eq) NH 2 OH·HCl and 0.98g (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com