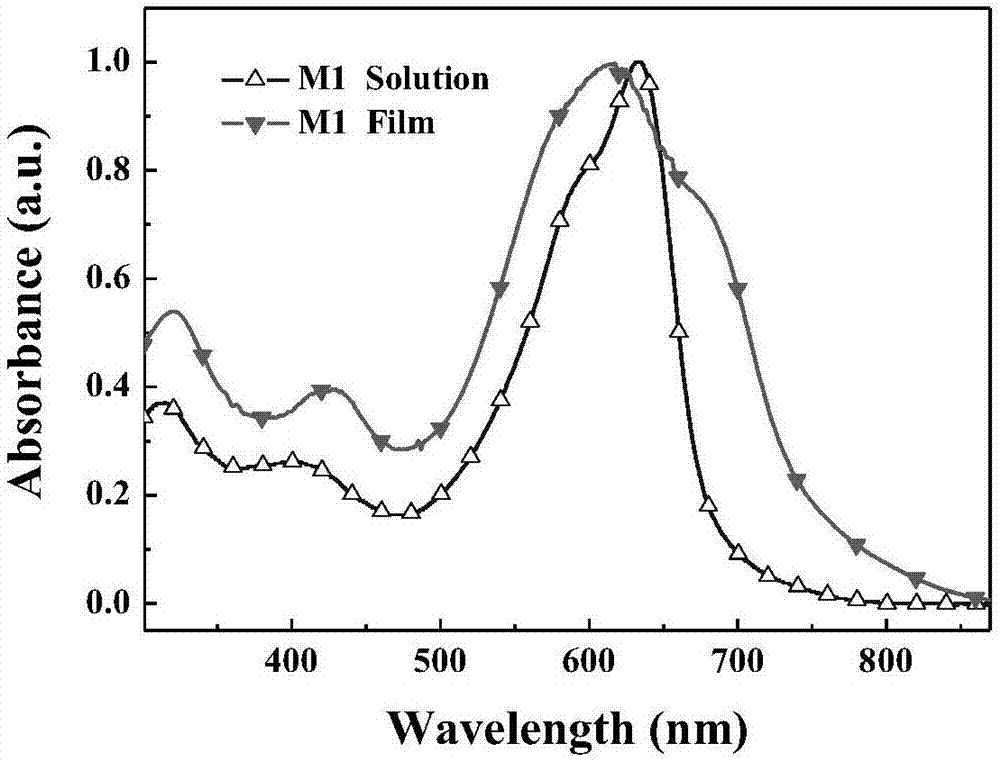
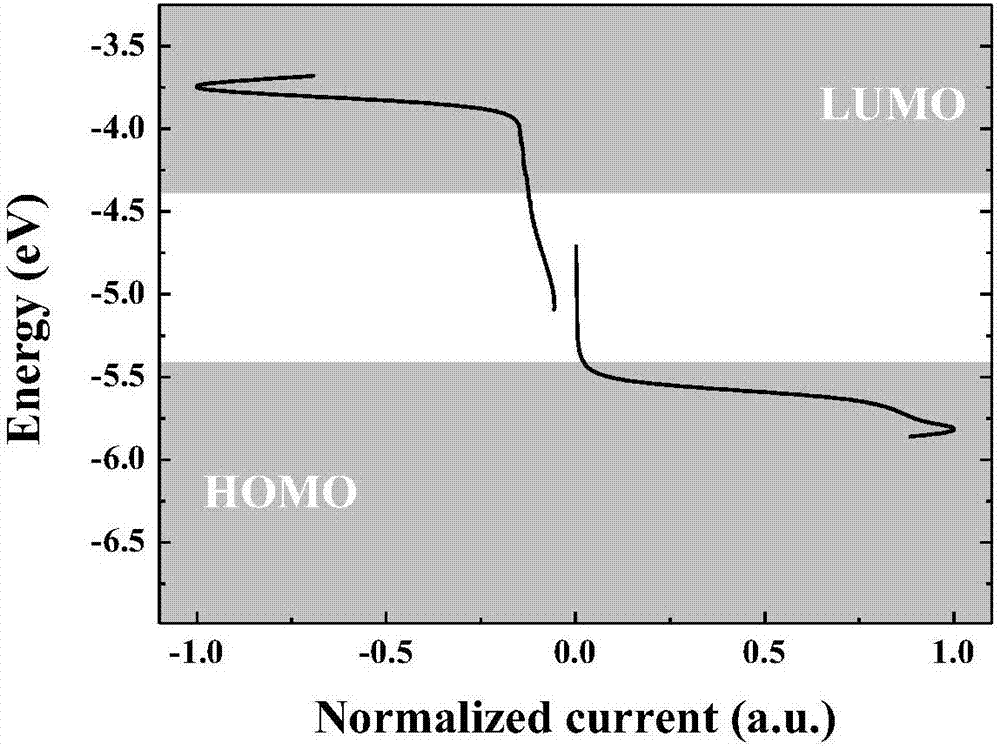
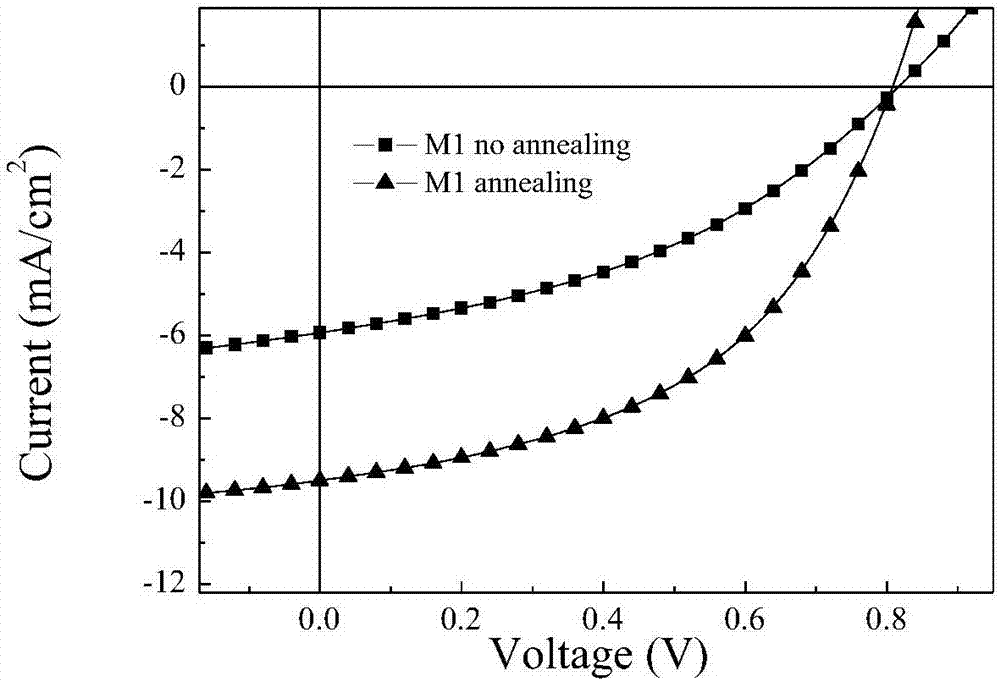
Two-dimensional conjugated benzodifuran organic micro-molecular photovoltaic material, and preparation method and application thereof
A benzodifuran, photovoltaic material technology, applied in photovoltaic power generation, organic chemistry, semiconductor/solid-state device manufacturing, etc., to achieve the effects of good photoelectric conversion efficiency, wide absorption spectrum, and good photoelectric conversion characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 4,8-Diisooctylthienyl-benzo(1,2-b; 4,5-b')-difuran-2,6-di(2,5-diisooctyl-3 , the preparation of 6-furyl-2-pyrrolopyrrole diketone) (M1)
[0057] Specific steps are as follows:
[0058] ①Synthesis of Compound 1
[0059] Under the protection of argon, 5 mL of anhydrous tetrahydrofuran was injected into 2-isooctylthiophene (3.92 mg, 20 mmol, purchased from Suzhou Nakai Technology Co., Ltd.), and 9.2 mL of n-butyllithium was added dropwise at 0 ° C. The system was raised to 50°C and stirred under reflux for 1.5 hours, and benzodifuran (1.5 mg, 8 mmol) was added to the reaction system. 50°C for another 1.5 hours, after cooling to room temperature, the SnCl 2 2H 2 O (14.4mg, 64mmol) was dissolved in 25.6mL of 10% hydrochloric acid solution and added dropwise to the above reaction solution. After continuing the reaction for 1.5 hours, the mixture was poured into water, extracted with dichloromethane, and the organic phase was dried over anhydrous magnesium sulfat...
Embodiment 2
[0077] Example 2 4,8-Diisooctylthienyl-benzo(1,2-b; 4,5-b')-difuran-2,6-di(2,5-diisooctyl-3 , the preparation of 6-furyl-2-pyrrolopyrrole diketone) (M1)
[0078] Specific steps are as follows:
[0079] ①Synthesis of Compound 1
[0080] Under argon protection, 5 mL of anhydrous tetrahydrofuran was injected into 2-isooctylthiophene (3.14 mg, 16 mmol, purchased from Suzhou Nakai Technology Co., Ltd.), and 9.2 mL of n-butyllithium was added dropwise at 0 ° C. The system was raised to 45°C and stirred under reflux for 1 hour, and benzodifuran (1.5 mg, 8 mmol) was added to the reaction system. 45°C for another 1 hour, after cooling to room temperature, the SnCl 2 2H 2 O (12.6mg, 56mmol) was dissolved in 25.6mL of 10% hydrochloric acid solution and added dropwise to the above reaction solution. After continuing the reaction for 1 hour, the mixture was poured into water, extracted with dichloromethane, and the organic phase was dried over anhydrous magnesium sulfate and filtered. ...
Embodiment 3
[0098] Example 3 4,8-Diisooctylthienyl-benzo(1,2-b; 4,5-b')-difuran-2,6-di(2,5-diisooctyl-3 , the preparation of 6-furyl-2-pyrrolopyrrole diketone) (M1)
[0099] Specific steps are as follows:
[0100] ①Synthesis of Compound 1
[0101] Under the protection of argon, 5 mL of anhydrous tetrahydrofuran was injected into 2-isooctylthiophene (4.70 mg, 24 mmol, purchased from Suzhou Nakai Technology Co., Ltd.), and 9.2 mL of n-butyllithium was added dropwise at 0 ° C. The system was raised to 55°C and stirred under reflux for 2 hours, and benzodifuran (1.5 mg, 8 mmol) was added to the reaction system. 55°C for another 2 hours, after cooling to room temperature, the SnCl 2 2H 2 O (16.2mg, 72mmol) was dissolved in 25.6mL of 10% hydrochloric acid solution and added dropwise to the above reaction solution. After continuing to react for 2 hours, the mixture was poured into water, extracted with dichloromethane, and the organic phase was dried over anhydrous magnesium sulfate and filt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com