Preparation of Sutherlandin-5-p-hydroxybenzoate, and applications of Sutherlandin-5-p-hydroxybenzoate in preparation of rheumatoid arthritis treatment drugs
A technology for rheumatoid arthritis and compounds, applied in the preparation of Sutherlandin-5-p-hydroxybenzoate and its application in the preparation of drugs for the treatment of rheumatoid arthritis, which can solve the problems of high treatment cost and heavy social burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1.Sutherlandin-5-p-hydroxybenzoate
[0026] Take the pearl plum medicinal material, crush it into the coarsest powder, add 20 times the amount of 95% ethanol, soak and extract 1 to 3 times, about 10 hours each time, combine the extracts, filter, dealcoholize and concentrate to about 20% ethanol concentration, and add samples On the AB-8 type weakly polar macroporous adsorption resin column, first use 20% ethanol solution to elute 5 column volumes to remove impurities, then use 95% ethanol to elute 4 column volumes, collect the 95% ethanol eluted part, After drying under reduced pressure, put on a 100-200 mesh silica gel column, use dichloromethane-methanol as the elution system, and carry out stage elution according to the methanol concentration from low to high, collect the eluted part with a methanol concentration of 20-30%, concentrate, and crystallize , filtered, and dried under reduced pressure to obtain coarse crystals of Sutherlandin...
Embodiment 2
[0027] The preparation of embodiment 2.Sutherlandin-5-p-hydroxybenzoate
[0028] Take the pearl plum medicinal material, crush it into the coarsest powder, add 20 times the amount of methanol, soak and extract 1 to 3 times, about 10 hours each time, combine the extracts, filter, dealcoholize and concentrate to an appropriate volume, and replenish water until the alcohol concentration is about 20% , add the sample to D-101 non-polar macroporous adsorption resin column, first use 20% methanol solution to elute 5 column volumes to remove impurities, then use 90% methanol to elute 4 column volumes, collect 90% methanol to wash The eluted part was dried under reduced pressure and placed on a 100-200-mesh silica gel column, using dichloromethane-methanol as the elution system, and eluted step by step according to the methanol concentration from low to high, and collected the eluted part with a methanol concentration of 20-30%. Concentrate, crystallize, filter, and dry under reduced ...
Embodiment 3
[0029] Example 3. Confirmation of the structure of Sutherlandin-5-p-hydroxybenzoate
[0030] 1. Instruments and materials
[0031] Jasco P-1020 digital polarimeter, Agilent TOF / 6500 high-resolution mass spectrometer, Shimadzu UV-2401 visible-ultraviolet spectrophotometer, NMR is Bruke Avance III 500NMR spectrometer, melting point analyzer is Yanaco MP53 (melting point is not corrected) ). Sutherlandin-5-p-hydroxybenzoate samples were prepared according to the steps in Example 1 above.
[0032] 2. Compound structure identification
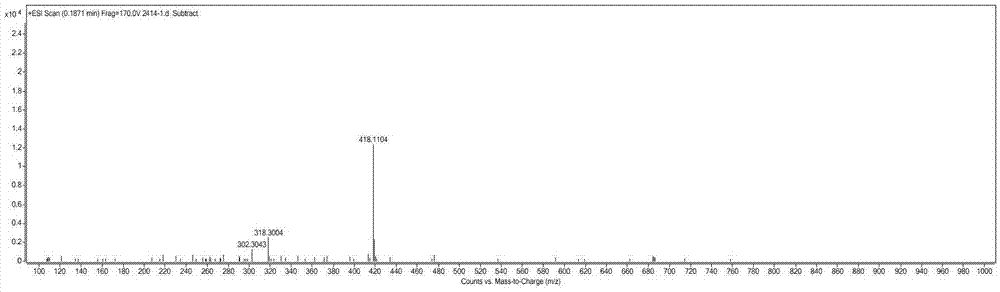
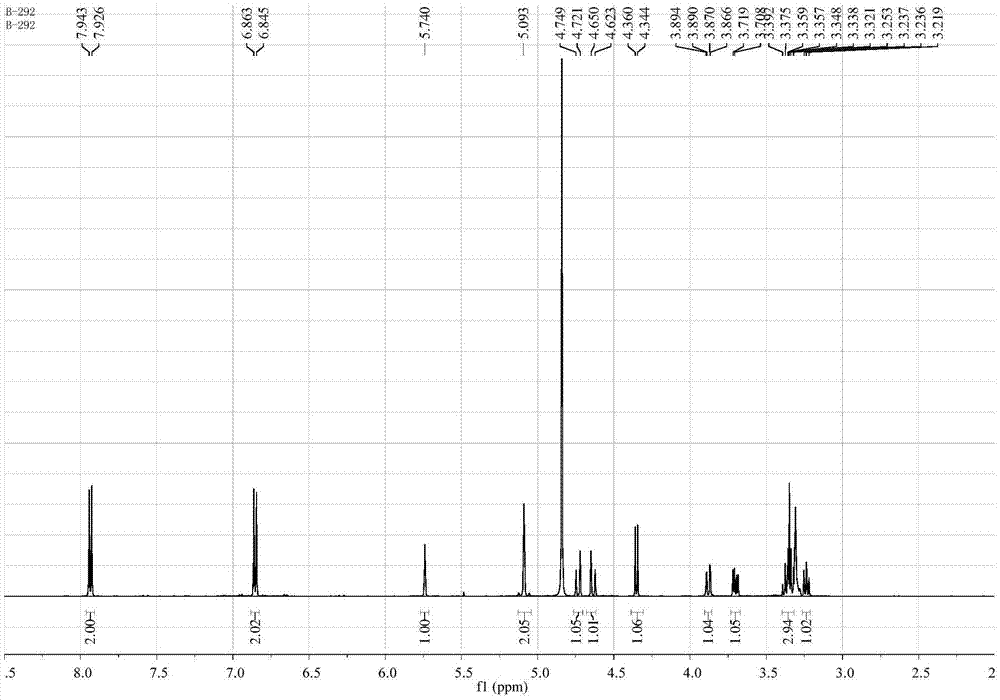
[0033] Colorless needle crystal (methanol), mp 79-81℃, easily soluble in dimethyl sulfoxide and methanol, slightly soluble in ethyl acetate, acetone, chloroform, water, insoluble in petroleum ether, Positive ion ESI-MS m / z: 396[M+H] + , 418[M+Na] + ; Negative ion ESI-MS m / z: 394[M-H] - , 430[M+Cl] - ,789[2M-H] - . Positive ion HR-ESI-MS m / z: measured value 418.1104[M+Na] + , the calculated value is 418.1114 (C 18 h 21 NO 9 Na[M+Na] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com