Salen catalyst using CN as axial ligand, and preparation method and application thereof
A catalyst and ligand technology, applied in the field of Salen catalyst and its preparation, can solve the problems of selectivity and polycarbonate chain link decline, affecting product performance, etc. the resulting effect of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
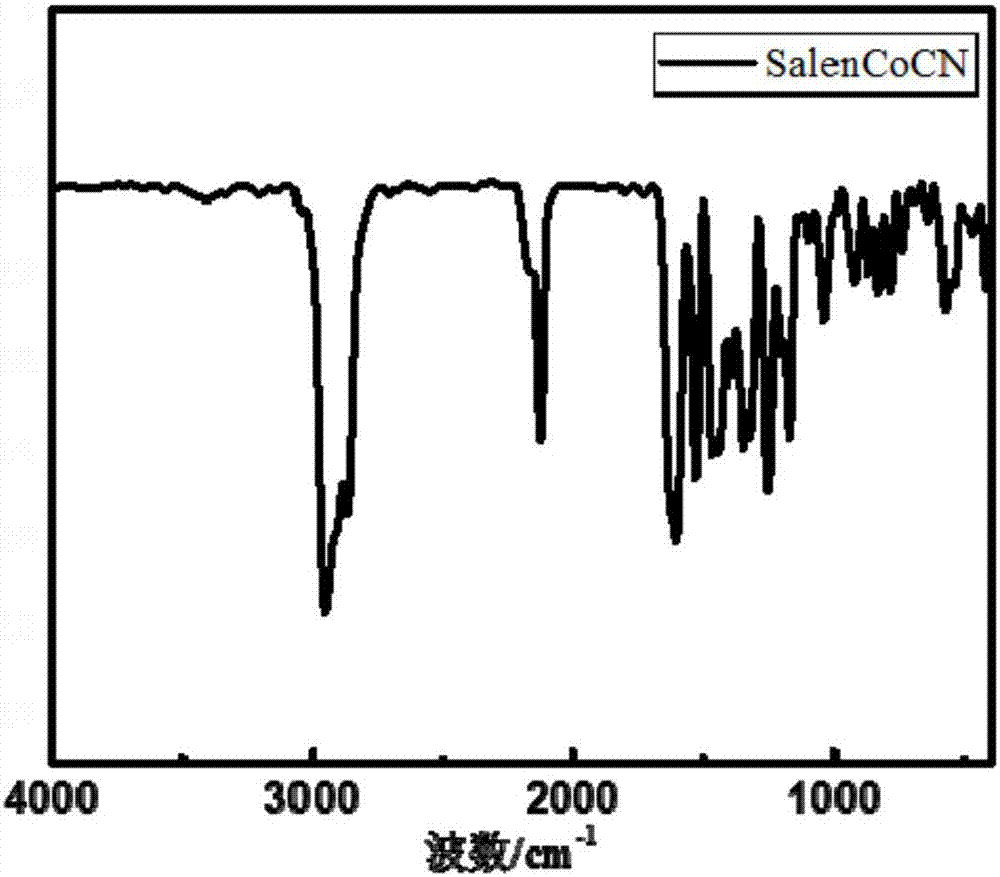
[0039] A SalenCo (III) CN catalyst, during preparation, at first cyclohexanediamine is split, then 2mol of 3,5-di-tert-butyl salicylaldehyde and 1mol of split cyclohexanediamine are synthesized into salicylaldimine, and then combined with anhydrous acetic acid The cobalt reaction is coupled with the metal center, and finally the SalenCo (II) Oxidation to trivalent and coupled with the axial ligand CN, the specific steps are:
[0040] (1) Splitting of cyclohexanediamine: Fix a 500mL three-neck flask in an oil bath, install a mechanical stirrer, a constant pressure dropping funnel, and a spherical reflux condenser at its three openings, and connect the pipelines After passing the condensed water, add 37.5g L-(+) tartaric acid and 100mL distilled water into the flask, turn on the mechanical stirrer, set the temperature of the oil bath to 70°C, measure 66.6mL of cis-trans cyclohexanediamine and pour it into the constant pressure drop Slowly add it dropwise into the above solutio...
Embodiment 2
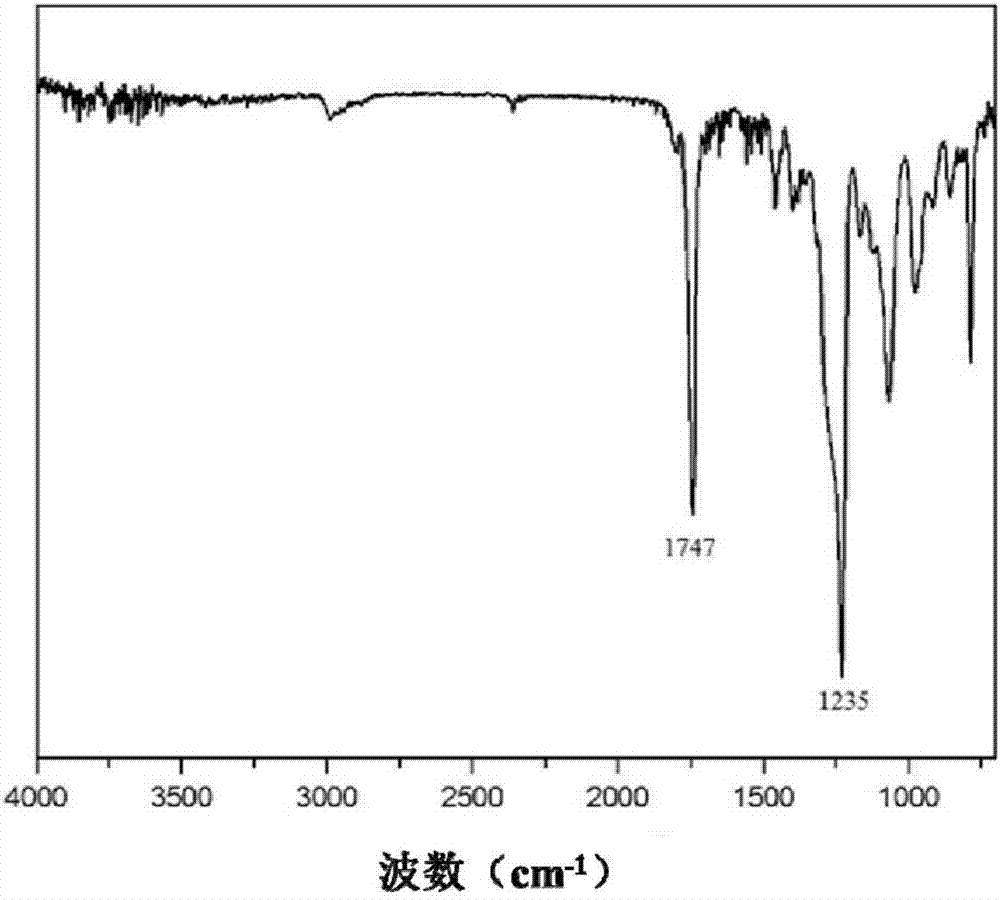
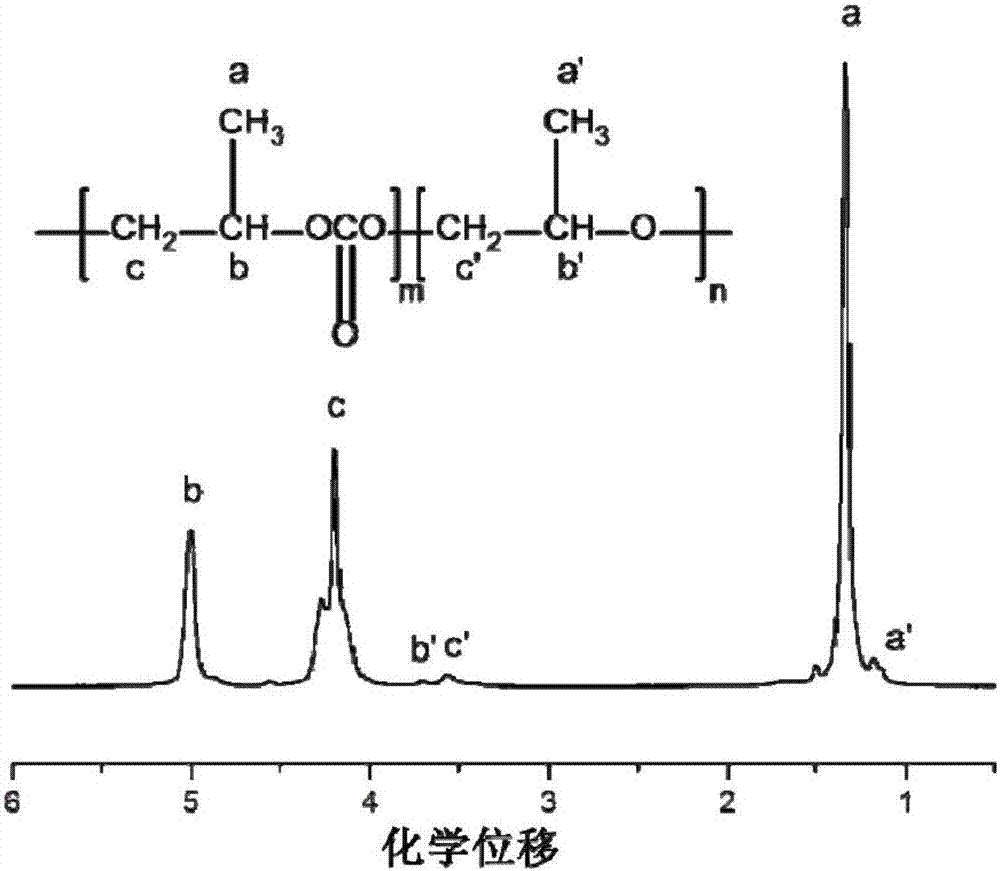
[0046] When the catalyst is used for the copolymerization reaction of carbon dioxide and epoxide to prepare polycarbonate, the specific steps are as follows: clean the reactor, vacuumize it, preheat it to 110°C, replace it with carbon dioxide three times, keep the vacuum state, and cool down the reactor to At 25°C, weigh the catalyst and propylene oxide, raise the temperature to the polymerization temperature, inject carbon dioxide to the set pressure, start mechanical stirring, and start timing. After the reaction is over, first turn off the mechanical stirring, lower the temperature to 25°C, open the pressure relief valve to release unreacted carbon dioxide, open the reaction kettle and quickly take it out for further 1 H NMR and GPC analysis of the reaction product tested, sample preparation test. Take out the remaining polymerization product and put it into a small beaker, add a small amount of CH 2 Cl 2 , Stir to dissolve it, then slowly pour it into a beaker containing...
Embodiment 3
[0049] In this example, 0.1mol Salen-Co was added to the reactor (III) CN and 0.1mol cocatalyst PPNCl, 14mL propylene oxide, set the reaction temperature to 50°C, feed 2.0MPa carbon dioxide, start mechanical stirring, and react for 2 hours. Polymerization result: TOF=378.59h -1 , selectivity = 94%, Mn = 27.7 kg / mol, PDI = 1.01.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com