Screening and reforming method for super-stable immunoglobulin variable domain, and applications thereof
A domain antibody, selected technology, applied in the direction of immunoglobulin, anti-animal/human immunoglobulin, compound screening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
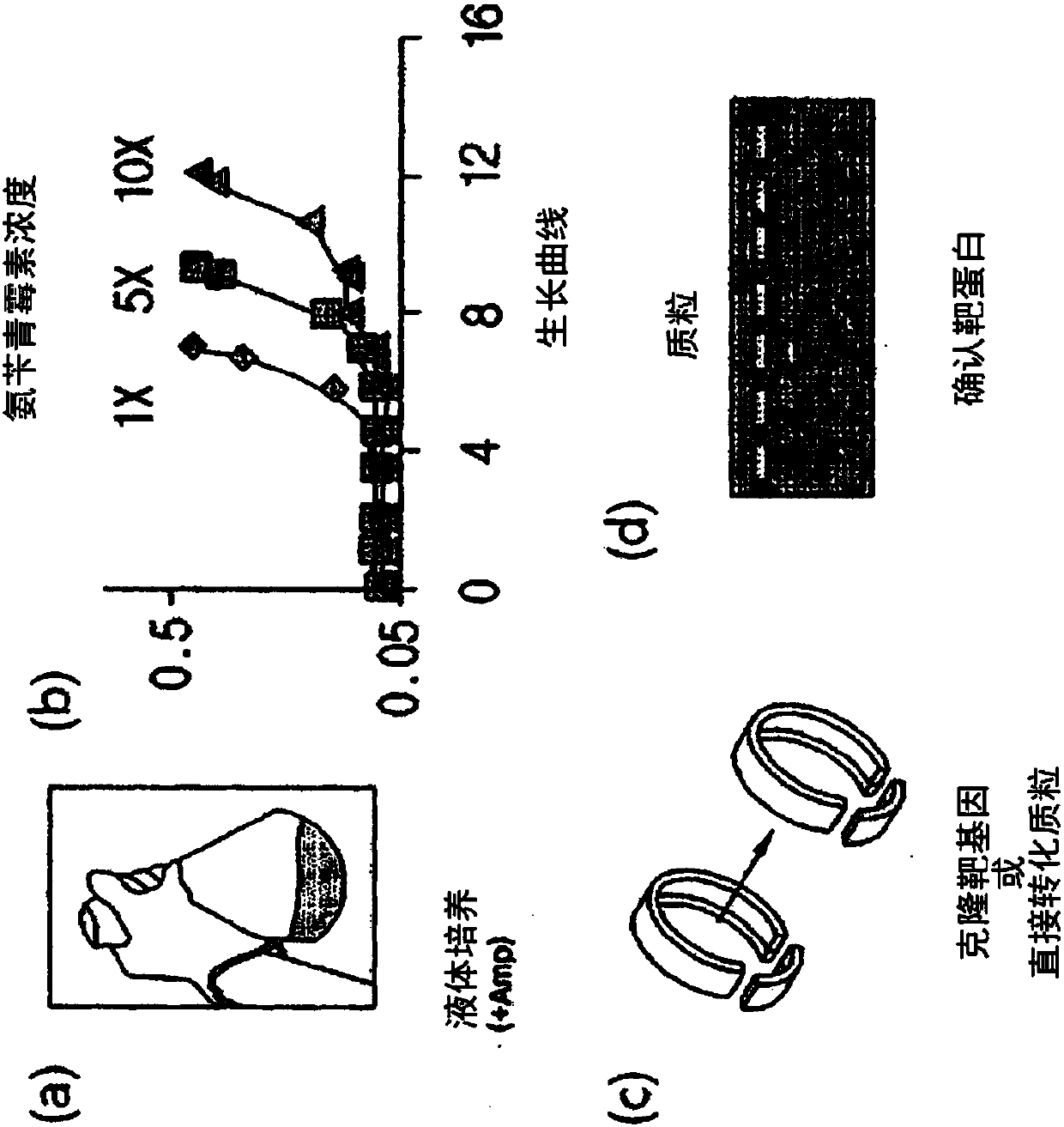
[0319] Example 1: Preparation of pET-TAPE for constructing TAPE system
[0320] In order to construct the twin arginine transporter (Tat)-associated protein engineering (TAPE) system, the pET9a vector was used to transform the pathway signal sequence of a Tat substrate protein TorA (Escherichia coli trimethylamine-N-oxide reductase) into , ssTorA, was linked to the N-terminus of the target protein and TEM-1 β-lactamase was linked to its C-terminus to make pET-TAPE.
[0321] However, the signal sequence that directs the protein to the Tat pathway is not limited to ssTorA, as it will be apparent to one of ordinary skill that the signal sequence of all Tat pathway proteins can be used, as described above. In addition, it is obvious to those of ordinary skill that, as the vector used, any vector satisfying the purpose of the present invention can be used, such as pET9a (New England Biolab), ΔpMA using an arabinose-inducible promoter (Korean Patent Application Laid-Open No. 1996-00...
Embodiment 2
[0329] Example 2: Preparation of Immunoglobulin Heavy Chain Variable Domain (VH) Libraries Derived from Human Germline
[0330] cDNA libraries were secured by reverse transcription of mRNA obtained from human liver, peripheral blood mononuclear cells (PBMC), spleen and thyroid.
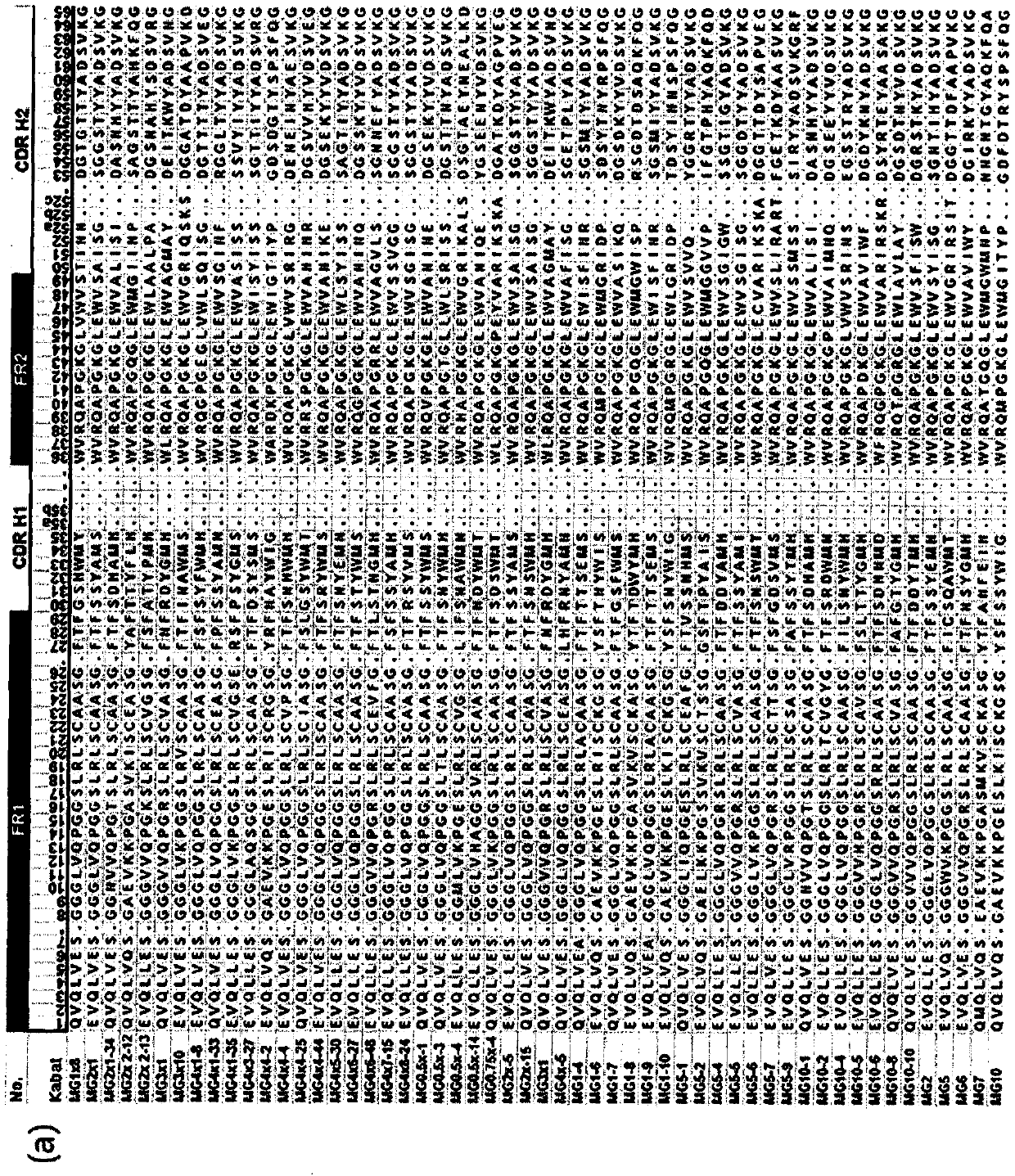
[0331] To thereby ensure the DNA sequence of the human immunoglobulin heavy chain variable region domain, a mixed primer shown in SEQ ID NO: 5 to 13 was designed to ensure that all human heavy chain variable domain genes are available in human germ cells Tie. Each ensured human heavy chain variable domain gene library was inserted between the NdeI and BamHI sites of pET-TAPE to complete with about 10 8 size library.
[0332] Specifically, cDNA was prepared from RNA extracted from human spleen, peripheral blood mononuclear cells, liver and thymus (Clontech, Madison, WI, US) by reverse transcription reaction. AMV reverse transcriptase and RNase inhibitors were purchased from Promega (Madison, WI, USA...
Embodiment 3
[0342] Example 3: Screening of VH libraries derived from human germlines by TAPE (Tat-associated protein engineering)
[0343] (1) Construction of VH library derived from human germline
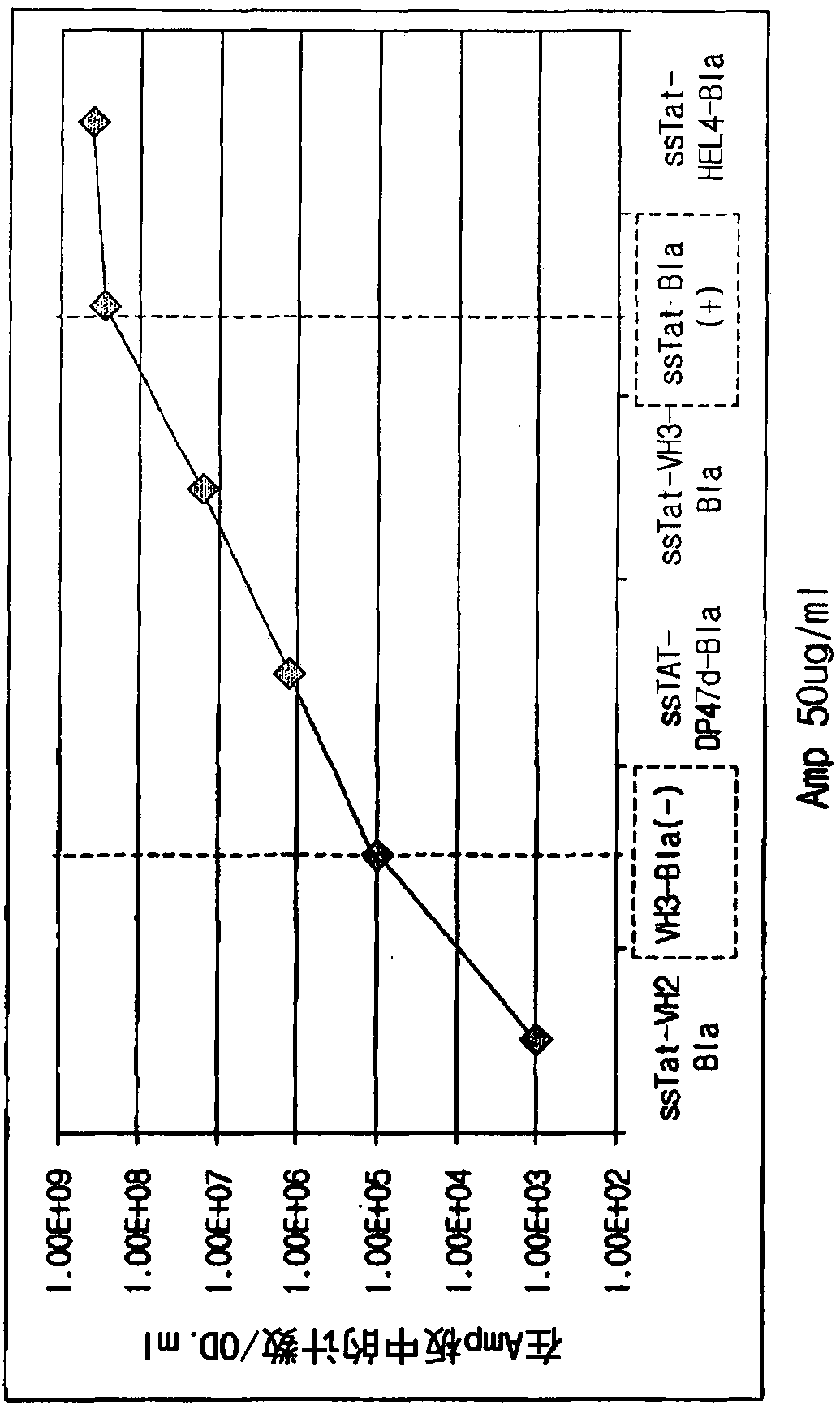
[0344] Transformation of Escherichia coli T7Express LysY / I with pET-TAPE library by electroporation q . Then, they were cultured at 37° C. in SOC medium for 1 hour, and then inoculated and cultured in LB medium containing 50 μg / ml of carbenicillin (1X). When the OD value of Escherichia coli was 0.6, the Escherichia coli was collected using centrifugation, and then the plasmid was isolated using a plasmid DNA purification kit (QIAGEN, Valencia, CA, USA), and then cut with restriction enzymes NcoI and BamHI. Cleavage genes include VH library and β-lactamase gene, which is used to rule out false positives that may appear in the subsequent liquid panning process. The pET-TAPE plasmid was also cut with restriction enzymes NcoI and BamHI. After cleavage, each DNA was purified using a gel extrac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com