Enhanced fluorescence probe for detecting carboxylesterase 1 (CES1) as well as preparation method and application thereof
A fluorescent probe, carboxylesterase technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of deviation of detection results, cumbersome preparation methods, limited application, etc., to promote the recovery of fluorescent signals , Excellent detection specificity, the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Embodiment 1: Preparation process 1 of probe compound 3-bromomethyl-2-oxo-2H-chromene-7-acetate
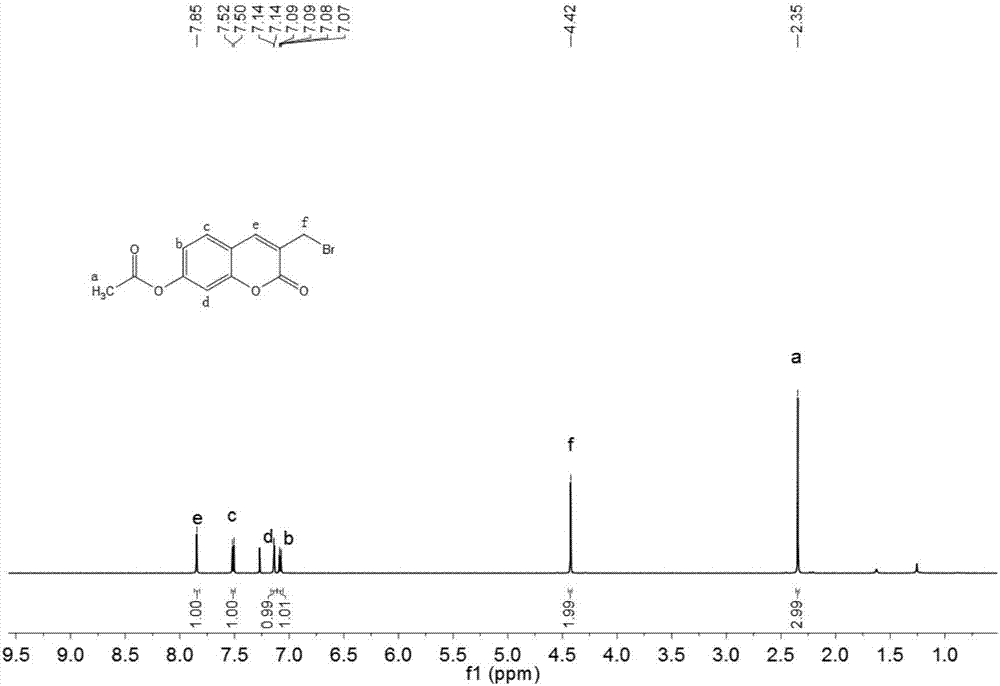
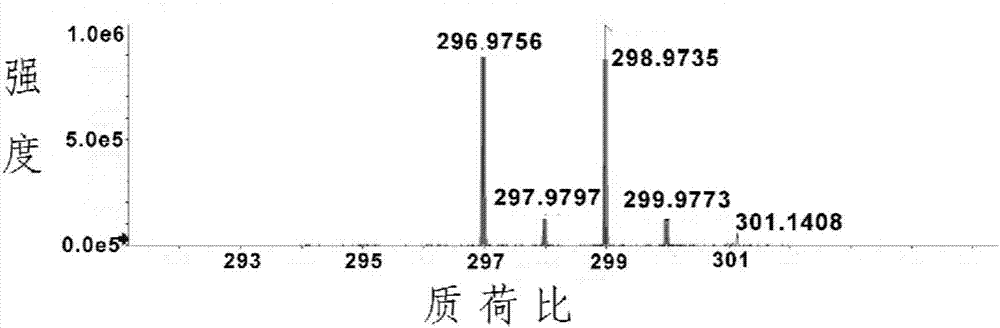
[0040] Dissolve 1090 mg of 3-methyl-2-oxo-2H-chromene-7-acetate (5 mmol), 890 mg of N-bromosuccinimide (5 mmol) and 0.82 mg of azobisisobutyronitrile in 30 mL of tetrachloro In carbonization, heat the mixed solution to reflux, control the reaction temperature to 80°C for reaction, stop the reaction after 4 hours of reaction, cool to room temperature, filter the mixed solution with suction, collect the organic filtrate, and remove the organic solvent by rotary evaporation to obtain a solid Purification by silica gel column chromatography (eluent: petroleum ether / ethyl acetate, V / V=4:1) gave 1115 mg of white solid (yield 75.1%). The product was characterized by proton nuclear magnetic resonance spectroscopy, and the results were as follows: figure 2 As shown, the characterization data are as follows: 1 H NMR (600MHz, CDCl 3 ): δ7.85(s,1H),7.51(d,J=8.5Hz,1H),7.14(d,J=2.1Hz,...
Embodiment 2
[0041] Embodiment 2: Preparation process 2 of probe compound 3-bromomethyl-2-oxo-2H-chromene-7-acetate
[0042] Dissolve 545 mg of 3-methyl-2-oxo-2H-chromene-7-acetate (2.5 mmol), 400 mg of N-bromosuccinimide (2.25 mmol) and 0.41 mg of azobisisobutyronitrile in 15 mL In carbon tetrachloride, heat the mixed solution to reflux, control the reaction temperature to 83°C for reaction, stop the reaction after 4.5 hours of reaction, cool to room temperature, suction filter the mixed solution, collect the organic filtrate, and remove the organic solvent by rotary evaporation. The obtained solid was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate, V / V=4:1) to obtain 487 mg of white solid (yield 72.9%). The characterization of the probe 3-bromomethyl-2-oxo-2H-chromene-7-acetate compound in this example is the same as that in Example 1.
Embodiment 3
[0043] Embodiment 3: Preparation process 3 of probe compound 3-bromomethyl-2-oxo-2H-chromene-7-acetate
[0044] Dissolve 1635mg of 3-methyl-2-oxo-2H-chromene-7-acetate (7.5mmol), 1270mg of N-bromosuccinimide (7.125mmol) and 1.23mg of azobisisobutyronitrile in 50mL In carbon tetrachloride, heat the mixed solution to reflux, control the reaction temperature to 85°C for reaction, stop the reaction after 5 hours of reaction, cool to room temperature, suction filter the mixed solution, collect the organic filtrate, and remove the organic solvent by rotary evaporation. The obtained solid was purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate, V / V=4:1) to obtain 1508 mg of white solid (yield 71.3%). The characterization of the probe 3-bromomethyl-2-oxo-2H-chromene-7-acetate compound in this example is the same as that in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com