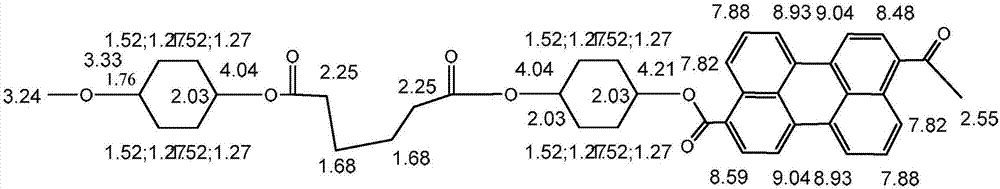
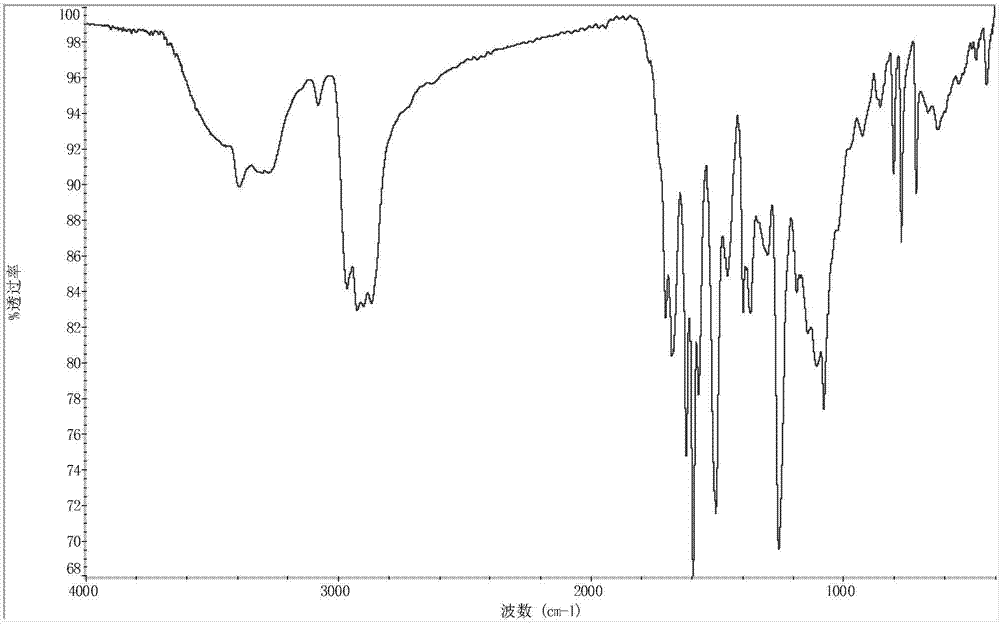
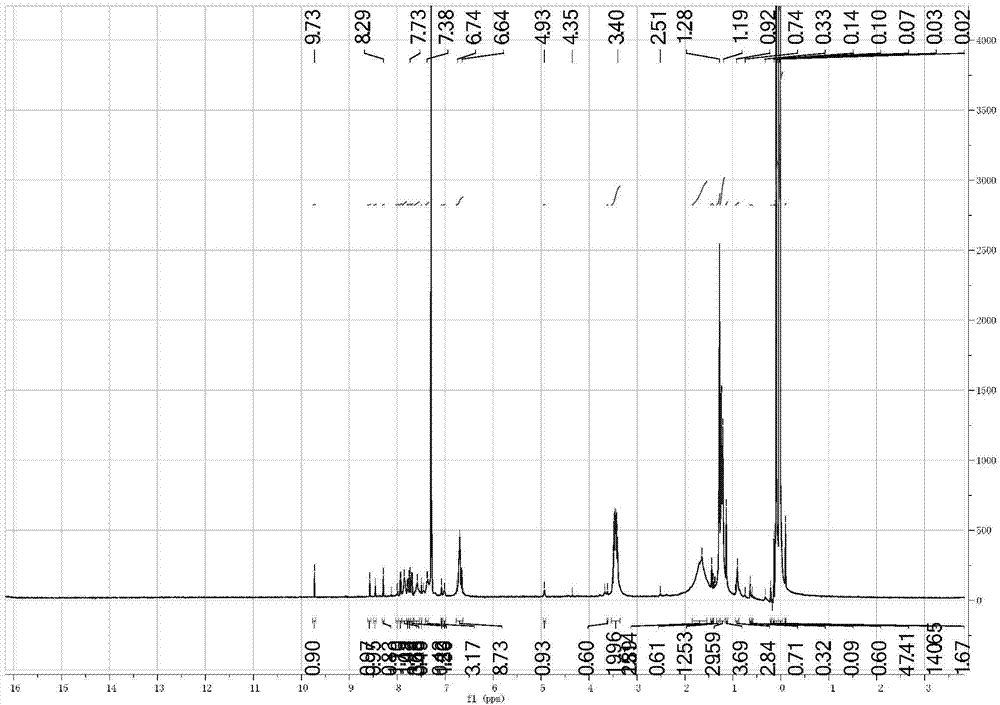
Alicyclic polyester type polymer dye for polypropylene plastic dyeing and preparation method thereof
A technology of cycloaliphatic polyester and polymer dyes, applied in the field of polymer dyes, can solve problems such as difficult to achieve dyeability, operability, mechanical properties, fast flow, low viscosity, etc., and improve chemical stability and durability Migration, increased compatibility, rich color effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Add 15.2g (0.05mol) perylene dye Fluorescent Yellow 8 and 14.4g (0.10mol) 1,4-cyclohexanedimethanol, 10mL toluene, and 0.06g titanium to a flask equipped with a reflux condenser and a water separator Tetrabutyl acid, stirred under the protection of nitrogen and heated to 180°C, refluxed water separation reaction for 4 hours, removed the heat when about 2mL of water was collected in the water separator, and after cooling to room temperature, 8g of hydroxyl-terminated fluorescent yellow was obtained. Glycol esters.
[0054] Add 64.9g (0.45mol) of 1,4-cyclohexanedimethanol, 73.0g (0.50mol) of adipic acid, 40mL of toluene, and 0.6g of tetrabutyl titanate to the above-mentioned dye alicyclic glycol ester , protected by nitrogen, stirred and heated to 180°C, refluxed for 8 hours to separate the water, remove the heat when about 18mL of water was collected in the water separator, use a rotary evaporator to vacuum remove the unreacted solvent and water-carrying agent, filter wi...
Embodiment 2
[0059] Add 26.7g (0.05mol) fluorescein dye solvent red 72 (CI45370:1) and 7.2g (0.05mol) 1,4-cyclohexanedimethanol to the flask equipped with reflux condenser and water separator, and 5mL of toluene, 0.03g of tetrabutyl titanate, stirring under the protection of nitrogen and heating up to 170°C, reflux for water separation and reaction for 2 hours, remove the heat when about 1mL of water is collected in the water separator, and after cooling to room temperature, the hydroxyl-terminated Solvent Red 72 Cycloaliphatic Glycol Ester.
[0060] Add 57.7g (0.40mol) of 1,4-cyclohexanedimethanol, 71.4g (0.43mol) of phthalic acid, 40mL of toluene, and 0.6g of titanic acid to the above solvent red 72 alicyclic glycol ester Tetrabutyl ester, nitrogen protection, stirring and heating to 190°C, reflux and water separation reaction for 9 hours, remove the heat when about 16mL of water is collected in the water separator, and use a rotary evaporator to vacuum remove unreacted raw materials, so...
Embodiment 3
[0062] Add 20.2g (0.075mol) of azo dye Acid Red 2 and 10.8g (0.075mol) of 1,4-cyclohexanedimethanol to a flask equipped with a reflux condenser and a water separator, and 5mL of toluene, 0.03g For tetrabutyl titanate, stir under nitrogen protection and heat up to 175°C, reflux for 2 hours, remove the heat when about 1mL of water is collected in the water separator, and cool to room temperature to obtain hydroxyl-terminated acid red 2 alicyclic di Acid esters.
[0063] Add 57.7g (0.40mol) of 1,4-cyclohexanedimethanol, 74.0g (0.43mol) of 1,4-cyclohexanedicarboxylic acid, and 40mL of toluene to the above acid red 2 alicyclic dibasic acid ester , 0.6g of tetra-n-butyl titanate, stirred under the protection of nitrogen gas and heated to 190 ° C, refluxed water separation reaction for 7 hours, when about 16mL of water was collected in the water separator, the heating was removed, and the unreacted product was removed by vacuum with a rotary evaporator The finished raw material and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| solid content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com