Layered perovskite catalyst for hydrogen generation from acetic acid autothermal reforming and preparation method
An autothermal reforming and perovskite-type technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of catalyst deactivation and achieve hydrogen High yield, improved reducibility, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
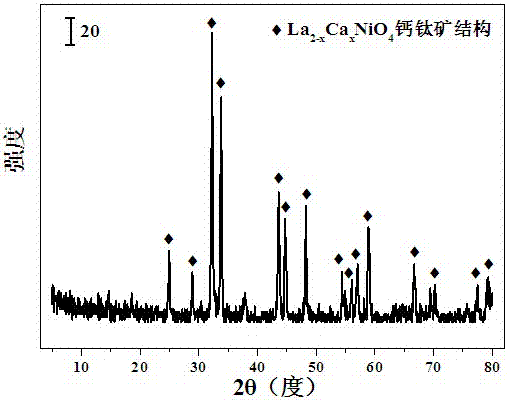
[0028] Weigh 8.6494 grams of La(NO 3 ) 3 ·6H 2 O and 2.9044 g of Ni(NO 3 ) 2 ·6H 2 O, add 30ml of deionized water to prepare solution #1; then weigh 6.2966 grams of citric acid C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to prepare solution #2; then weigh 1.8599 grams of ethylene glycol (CH 2 Oh) 2 ; Slowly add solution #1 and ethylene glycol dropwise to solution #2, and stir at 70°C for 4 hours, the solution gradually turns into a colloid, and transfer to a drying oven to dry at 105°C for 12 hours. Put the dried sample into a porcelain boat, put it into a tube furnace, raise the temperature to 700°C at a rate of 10°C / min, keep it at 700°C for 6 hours, and observe it with a scanning electron microscope and an X-ray diffractometer. The catalyst CDUT-LN with a layered perovskite structure was obtained with a molar composition of La 2 NiO 4 , and its typical XRD spectrum is attached figure 1 shown. The weight composition of the catalyst is as follows: the conten...
Embodiment 1
[0032] Weigh 5.8979 grams of La(NO 3 ) 3 ·6H 2 O,, 3.908 g of Ni (NO 3 ) 2 ·6H 2 O and 3.2165 g of Ca(NO 3 ) 2 4H 2 O, add 30ml of deionized water to make solution #1. Weigh 8.5863 g of C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to make solution #2. Weigh again 2.5362 grams of (CH 2 Oh) 2 . Slowly add solution #1 and ethylene glycol dropwise to solution #2, and stir at 70°C for 4 hours, the solution gradually turns into a colloid, and transfer to a drying oven to dry at 105°C for 12 hours. Put the dried sample into a porcelain boat, put it into a tube furnace, raise the temperature to 700°C at a rate of 10°C / min, keep it at 700°C for 6 hours, and obtain a CDUT with a layered perovskite structure- LC10N catalyst with a molar composition of LaCaNiO 4 , its XRD spectrum is attached figure 1 As shown, the results show that the layered perovskite structure is still formed after the addition of additive Ca. The weight composition of the catalyst is as follows...
Embodiment 2
[0035] Weigh 3.6045 grams of La(NO 3 ) 3 ·6H 2 O,, 4.8414 grams of Ni (NO 3 ) 2 ·6H 2 O and 5.8973 g of Ca(NO 3 ) 2 4H 2 O, add 30ml of deionized water to make solution #1. Weigh out 10.4965 g of C 6 h 8 o 7 ·H 2 O, add 30ml of deionized water to make solution #2. Weigh again 3.1004 grams of (CH 2 Oh) 2 . Slowly add solution #1 and ethylene glycol dropwise to solution #2, and stir at 70°C for 4 hours, the solution gradually turns into a colloid, and transfer to a drying oven to dry at 105°C for 12 hours. Put the dried sample into a porcelain boat, put it into a tube furnace, raise the temperature to 700°C at a rate of 70°C / min, keep it at 700°C for 6 hours, and obtain a CDUT with a layered perovskite structure- LC15N catalyst with a molar composition of La 0.5 Ca 1.5 NiO 4 , by XRD test, the results show that the catalyst forms a layered perovskite structure, accompanied by a small amount of calcium oxide phase. The weight composition of the catalyst is as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com