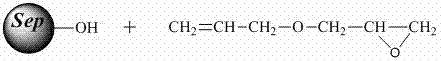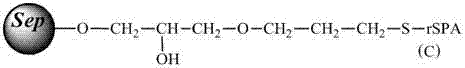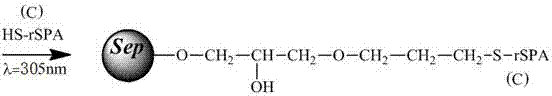Immune adsorption material for fixed point immobilizing protein A and preparation method thereof
An immunoadsorption material and protein technology, applied in the direction of solid adsorbent liquid separation, separation methods, chemical instruments and methods, etc., can solve the problems of reduced adsorption capacity, low antibody adsorption capacity, and inability to remove pathogenic antibodies by one-time perfusion , to improve the adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of recombinant protein A with cysteine
[0030] The coding sequence of the recombinant protein A with cysteine is obtained by artificial synthesis, and the sequence connects the B domains in the protein A coding sequence (SEQ.ID.NO: 1) to form 5 B domains Concatenate, the recognition sequence of restriction endonuclease NdeI located at the 5' end of the sequence; a codon tgt encoding cysteine and 6 histidine codons caccaccaccaccaccac located at the 3' end of the sequence XhoI recognition sequence.
[0031] SEQ.ID.NO.1
[0032]CATATGGTAGACaacaaattcaacaaagaacaacaaaatgctttctatgaaatcttacatttacctaacttaaacgaagaacaacgcaatggtttcatccaaagcctGaaagatgacccaagccaaagcgctaaccttttagcagaagctaaaaagctaaatgatgcGcaagcaccaaaaGTAGACaacaaattcaacaaagaacaacaaaatgctttctatgaaatcttacatttacctaacttaaacgaagaacaacgcaatggtttcatccaaagcctGaaagatgacccaagccaaagcgctaaccttttagcagaagctaaaaagctaaatgatgcGcaagcaccaaaaGTAGACaacaaattcaacaaagaacaacaaaatgctttctatgaaatcttacatttacctaacttaaacga...
Embodiment 2
[0035] Example 2: Preparation of Immunoadsorbent Material
[0036] Activation of Sepharose
[0037] Add 60 ml of 2.5 M sodium hydroxide aqueous solution containing 0.2% sodium borohydride to 30 ml of drained agarose gel Sepharose 6FF, mix well, shake in a shaker (50-200rpm) for 2 hours, and the reaction temperature is 40°C, and then add 30ml of allyl glycidyl ether to continue the reaction for 6 hours. After the reaction is complete, rinse thoroughly with distilled water and drain to obtain agarose gel with alkenyl groups.
[0038] Immobilization of protein A
[0039] Dissolve 210 mg of protein A in Example 1 in 30 ml of 0.1 mol / mL phosphate buffer solution (pH=7.4), and then add it to 30 ml of the above-mentioned activated agarose gel (that is, each ml of agarose gel corresponds to 7 mg of protein A), under the irradiation of ultraviolet light with a wavelength of 305nm, shake at room temperature (50-200rpm) for about 10 hours, recover the unreacted protein A solution, was...
Embodiment 3
[0040] Embodiment three: the preparation of adsorption material
[0041] Activation of Sepharose
[0042] Operate in the same way as in Example 2 to obtain agarose gel with alkenyl groups.
[0043] Immobilization of protein A
[0044] Dissolve 180 mg of protein A in Example 1 in 30 ml of 0.1 mol / mL phosphate buffer solution (pH=7.4), and then add it to 30 ml of the above-mentioned activated agarose gel (that is, each ml of agarose gel corresponds to 6 mg of protein A), under the irradiation of ultraviolet light with a wavelength of 305nm, shake at room temperature (50-200rpm) for about 16 hours, recover the unreacted protein A solution, wash the adsorbent with water several times, and drain it. Add 30ml of PBS solution containing 0.2M mercaptoethanol, shake at room temperature (50-200rpm) for 4 hours, wash with distilled water for several times, and drain to obtain an adsorption material for immobilizing protein A. The fixed amount of protein A detected by biuret method was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com