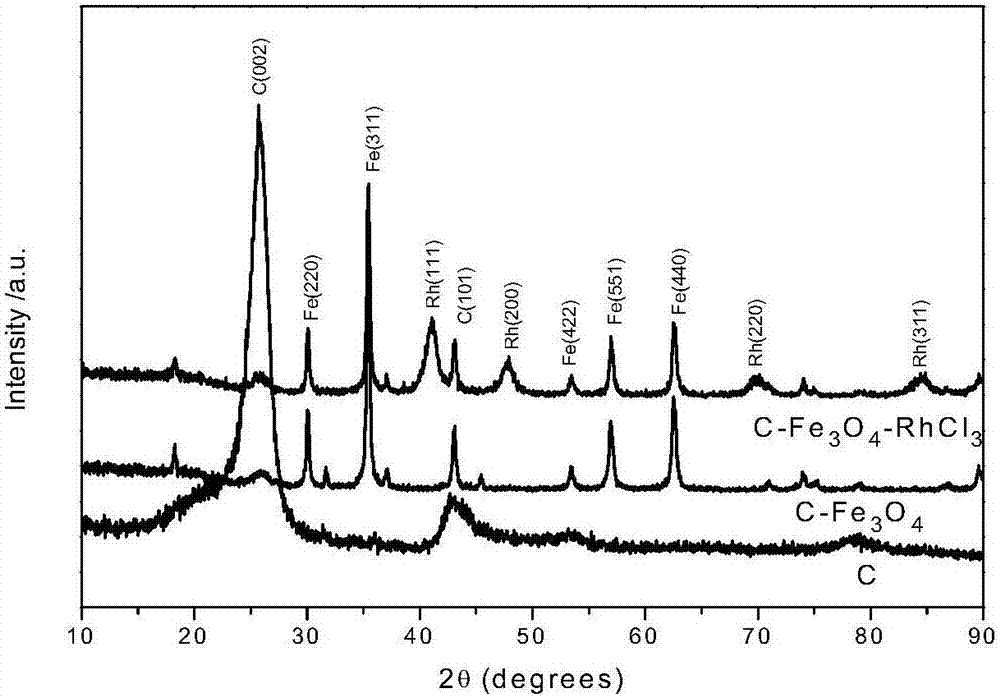
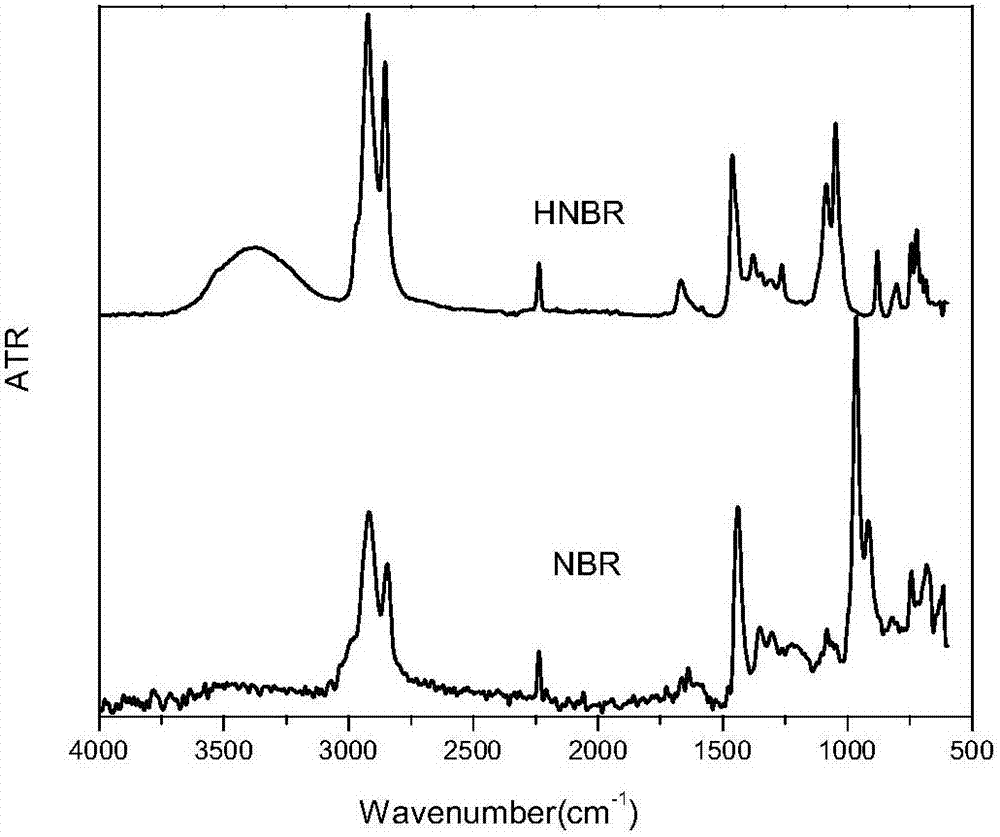
Preparation of magnetic carbon nanotube supported rhodium catalyst and application thereof in selective hydrogenation of NBR (nitrile butadiene rubber)
A technology of magnetic carbon nanotubes and rhodium catalysts, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve HNBR production cost increase, hydrogenation selectivity is not as good as rhodium, HNBR performance reduction, etc. problem, to achieve the effect of good recovery and recycling performance, good selectivity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of magnetic carbon nanotube supported rhodium catalyst in the present embodiment is as follows:
[0028] Step 1: Preparation of magnetic carbon nanotubes
[0029] Take 0.3g of carbon nanotubes in 75ml of ethylene glycol and sonicate for 5 hours, then add 2.0g of ferric chloride and 4.2g of sodium acetate to the above solution, stir magnetically for 2 hours, put it in a hydrothermal reaction kettle after homogenization, and put it in water at 200°C After thermal reaction for 8 hours, after the completion of the reaction, magnetic separation removes unmagnetized carbon nanotubes to obtain magnetic carbon nanotubes (C-Fe 3 o 4 ).
[0030] Step 2: Preparation of magnetic carbon nanotube-supported rhodium catalyst
[0031] Take 0.4g of C-Fe prepared in step 1 3 o 4 Ultrasonic dispersion in ethanol solvent for 3 hours, then add 100mg rhodium trichloride, react at 120°C for 30 minutes, cool to room temperature after the reaction, and magnetically se...
Embodiment 2
[0035] The reaction time of hydrogenating NBR was set to 2h, 4h, 6h, 8h, 10h, and 12h respectively, and other preparation parameters were kept unchanged to study the influence of different catalytic hydrogenation times on the hydrogenation degree of NBR. The experimental results are shown in Table 1.
[0036] Table 1 Effect of different catalytic hydrogenation time on NBR hydrogenation degree
[0037] Time(h) 2 4 6 8 10 12 HD(%) 69.32 80.63 94.26 98.17 98.25 98.54
[0038] It can be seen from Table 1 that with the increase of time, the hydrogenation degree of NBR is constantly increasing, but a good effect has been achieved at about 8h.
Embodiment 3
[0040] The reaction pressure of hydrogenated NBR in Example 1 was changed to 1MPa, 2MPa, 3MPa, 4MPa, 5MPa, and other preparation parameters were kept unchanged to study the influence of different reaction pressures on the hydrogenation degree of NBR. The experimental results are shown in Table 2.
[0041] Table 2 The influence of different reaction pressures on the hydrogenation degree of NBR
[0042] Pressure (MPa) 1 2 3 4 5 HD(%) 68.85 83.38 87.56 98.17 98.25
[0043] It can be seen from Table 2 that with the increase of hydrogenation pressure, the hydrogenation degree of NBR is constantly increasing, but it has achieved good results at around 4MPa.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com