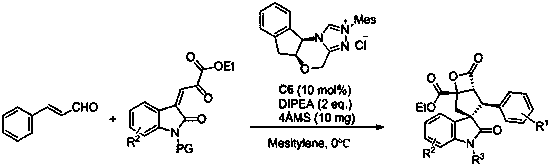
A kind of spiro-oxindole cyclopentane and β-lactone compound synthetic method
A spiro-oxindole cyclopentane and a synthesis method technology, which is applied in the field of synthesis of spiro-oxindole compounds, can solve problems such as difficulty in obtaining, and achieve simple operation, excellent diastereoselectivity, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] The chemical structural formula of nitrogen heterocyclic carbene is as follows:
[0031]
[0032] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, 1a (19.8 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence, and add 0.5 mL of Toluene was reacted at 0°C for 24 hours. The reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product 3a (21.1 mg) as a white solid. The rate was 54%, >99 / 1 dr, 91% ee; antibacterial drugs could be prepared.
[0033] Add nitrogen heterocyclic carbene (1.85 mg, 0.005 mmol) as a catalyst, and 1a (19.8 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (5 mg) in turn into the reaction flask, add 0.5 mL mesitylene , reacted at 0°C for 24 hours, and the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product 3a (15.2 mg), white so...
Embodiment 2
[0037]
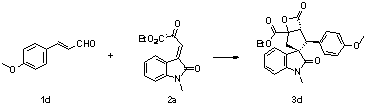
[0038] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, 1b (24.3 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence, and add 0.5 mL of Toluene was reacted at 0°C for 24 hours, and the reaction system was subjected to simple column chromatography (eluent: ethyl acetate: petroleum ether = 1:5) to obtain the target product 3b (23.9 mg) as a white solid. The rate was 57%, >99 / 1 dr, 91% ee, and antineoplastic drugs could be prepared.
[0039] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (24.3 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol) in sequence in the reaction flask, add 0.5 mL of mesitylene, at 0 °C After 24 hours of reaction, the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:5) to obtain the target product 3b (20.8 mg), a white solid, with a yield of 51%, >99 / 1 dr, 88% ee.
[0040] The product 3b was analyzed and the results are as foll...
Embodiment 3
[0042]
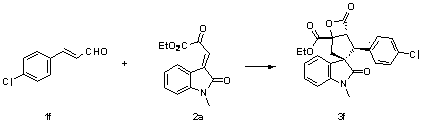
[0043] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (26.5 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence in the reaction flask, add 0.5 mL of Toluene was reacted at 0°C for 24 hours. The reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10) to obtain the target product 3c (28.3 mg) as a white solid. The rate was 65%, >99 / 1 dr, 86% ee, and enzyme inhibitors could be prepared.
[0044] Add nitrogen heterocyclic carbene (3.7 mg, 0.01 mmol) as a catalyst, and 1b (26.5 mg, 0.15 mmol), 2a (26 mg, 0.1 mmol), 4Å molecular sieves (10 mg) in sequence in the reaction flask, add 0.5 mL tetrahydrofuran, After reacting at 0°C for 24 hours, the reaction system was subjected to simple column chromatography (eluent: ethyl acetate:petroleum ether=1:10) to obtain the target product 3c (20.6mg), a white solid, and the yield was 47%, >99 / 1 dr, 81% ee.
[0045] The produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com