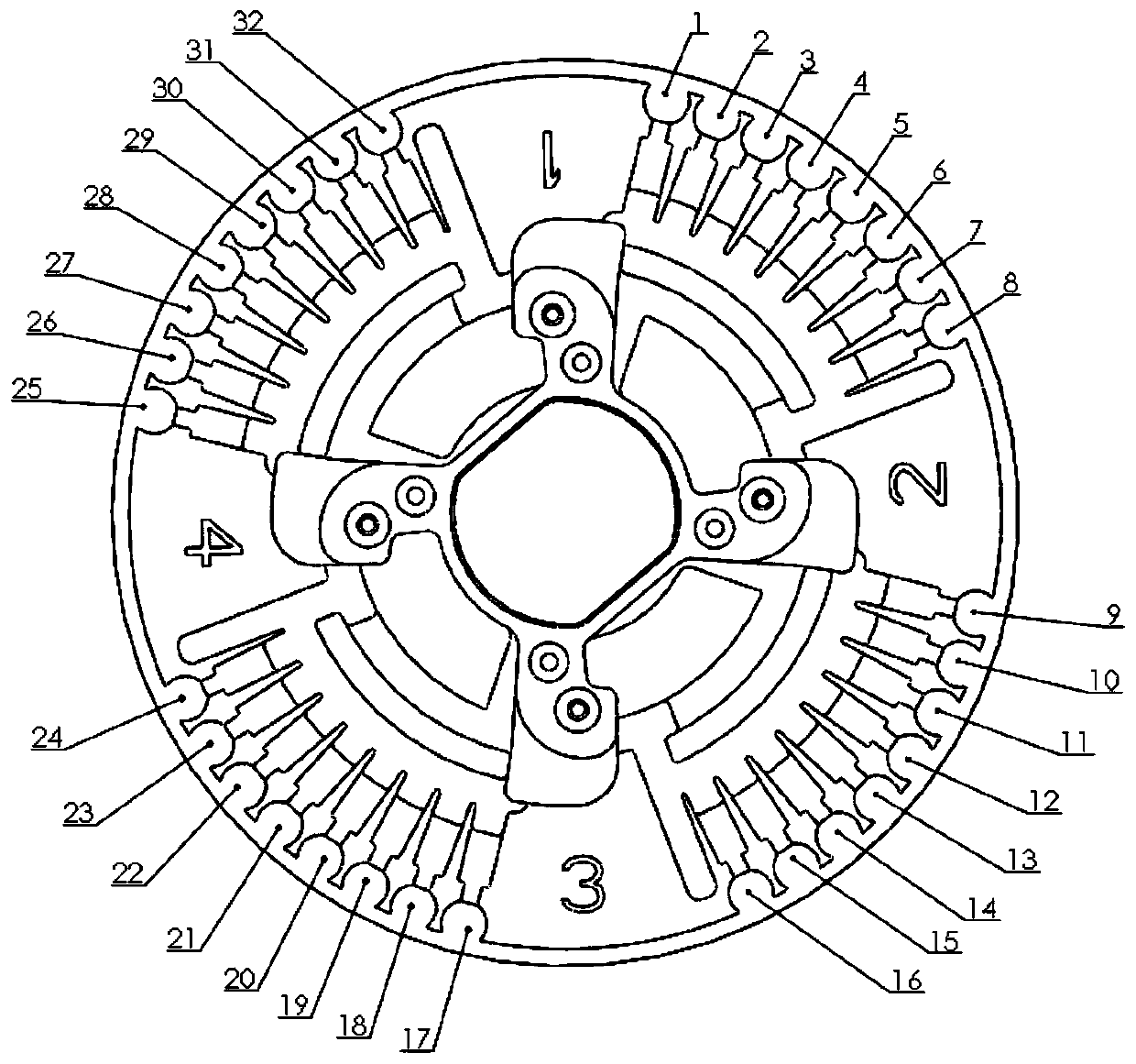
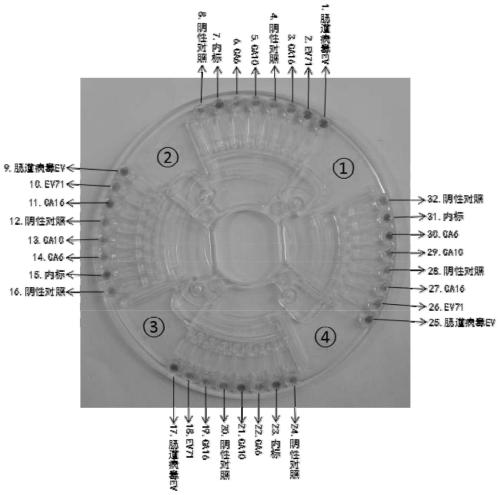
A kind of lamp primer composition and related application for detecting hand, foot and mouth disease-related pathogens
A primer composition and hand, foot and mouth disease technology, applied in the field of infectious disease diagnosis, can solve the problems of expensive detection equipment, few detection targets, low sensitivity, etc., and achieve high accuracy, good specificity, and improved detection speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Design and preparation of the LAMP primer composition for detecting hand-foot-mouth disease-related pathogens
[0029] 1.1 Sequence acquisition:
[0030] (1) Obtaining the sequence of the enterovirus 5'UTR region: download the nucleic acid sequence of the enterovirus 5'UTR region (as shown in SEQ ID NO.26) from the GeneBank public database.
[0031] (2) Acquisition of the VP1 gene sequence: download the enterovirus EV71 type gene sequence (as shown in SEQ ID NO.27), the CA16 type gene sequence (as shown in SEQ ID NO.28), and the CA10 type gene sequence from the GeneBank public database. The gene sequence (as shown in SEQ ID NO.29) and the VP1 gene sequence of CA6 type (as shown in SEQ ID NO.30).
[0032] (3) Acquisition of the internal reference HBB gene sequence: download the HBB gene nucleic acid sequence (as shown in SEQ ID NO.25) from the GeneBank public database.
[0033] 1.2 Primer design
[0034] (1) Specific gene primers: compare the above-mentioned...
Embodiment 2
[0048] Example 2 Preparation and use of a kit for detecting enterovirus-associated pathogens
[0049] The clinical samples of enterovirus EV, enterovirus EV71, enterovirus CA16, enterovirus CA10, enterovirus CA6, influenza A virus and hepatitis B virus in the following examples are all from Fudan University Affiliated Children's Hospital.
[0050] 2.1 Preparation of kits for detection of enterovirus-associated pathogens
[0051] The kit for detecting enterovirus-associated pathogens provided by the present invention consists of the following:
[0052] 2.1.1 Isothermal amplification buffer
[0053] The solvent of the constant temperature amplification buffer is water, and the solute and concentration are as follows: 12mM dNTPs, 10×Isothermal Amplification reaction buffer, 150mM MgSO4 aqueous solution, and 150μM HNB.
[0054] 2.1.2 Constant temperature amplification enzyme solution
[0055] The solvent of the constant temperature amplification enzyme solution is water, and t...
Embodiment 3
[0095] Example 3: Detecting the Sensitivity of the Thermostatic Amplification Microfluidic Chip
[0096] Using the isothermal amplification microfluidic chip prepared in 2.1 of Example 2 to conduct a sensitivity experiment
[0097] Enterovirus universal type, enterovirus EV71 type, enterovirus CA16 type, enterovirus CA10 type, and enterovirus CA6 type nucleic acids prepared in 2.2.2 of Example 2 were diluted respectively and mixed to obtain a new mixed system. In the new mixed system, the nucleic acid concentrations of enterovirus universal type, enterovirus EV71 type, enterovirus CA16 type, enterovirus CA10 type, and enterovirus CA6 type are all 102 TU / μl, and the resulting mixed system is named For mixed nucleic acid LP.
[0098] The mixed nucleic acid LP was mixed with the constant temperature amplification system, and then injected into the constant temperature amplification microfluidic chip, and the constant temperature amplification reaction was carried out according t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com