Method for determining contents of morphine and glycyrrhizic acid in compound liquorice tablet
A technology of compound licorice tablets and glycyrrhizic acid, which is applied to measuring devices, instruments, scientific instruments, etc., can solve the problems of complicated operation, low precision, long time consumption, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
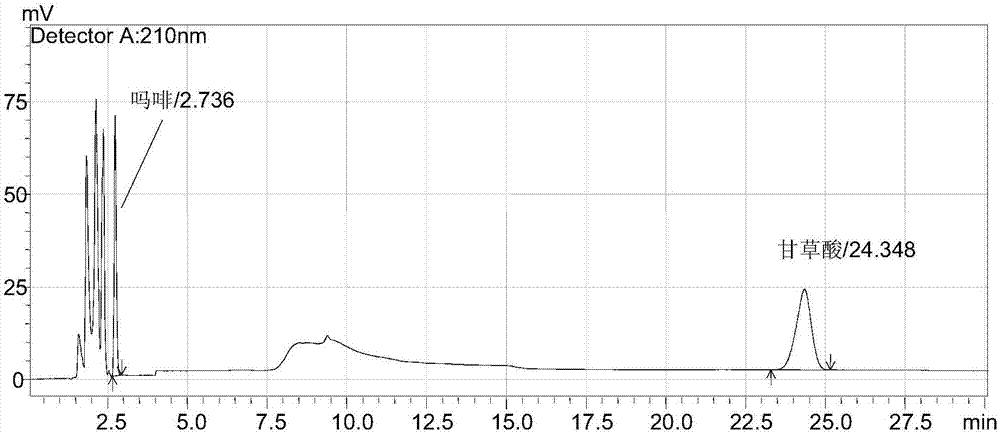
[0047] 1. Chromatographic conditions
[0048] Chromatographic column: SUPERIOREX (Shiseido) MG C18 250×4.6mm 5μm, column temperature: 30℃~40℃, flow rate: 1.3ml / min~1.7ml / min, detection wavelength: 210nm, 250nm, injection volume: 10μl~25μl .
[0049] 2. Solution Preparation
[0050] 2.1 Mobile phase: phosphate buffer and acetonitrile
[0051] 2.2 Phosphate buffer: Take 6.8g of potassium dihydrogen phosphate, dissolve it in an appropriate amount of water, add 1.1g of sodium heptanesulfonate and 2ml of triethylamine, adjust the pH to 4.9±0.1 with 20% phosphoric acid solution, and dilute to 1000ml with water.
[0052] 2.3 Diluent: Take 50ml of glacial acetic acid, add water to 500ml, mix well, add 500ml of methanol, shake well, and you get it.
[0053] 2.4 Test solution: Take 20 tablets of this product, accurately weigh, grind finely, accurately weigh 0.15881g, 0.15675g, 0.15656g, put them in 50ml measuring bottles respectively, add appropriate amount of diluent, ultrasonic for...
Embodiment 2
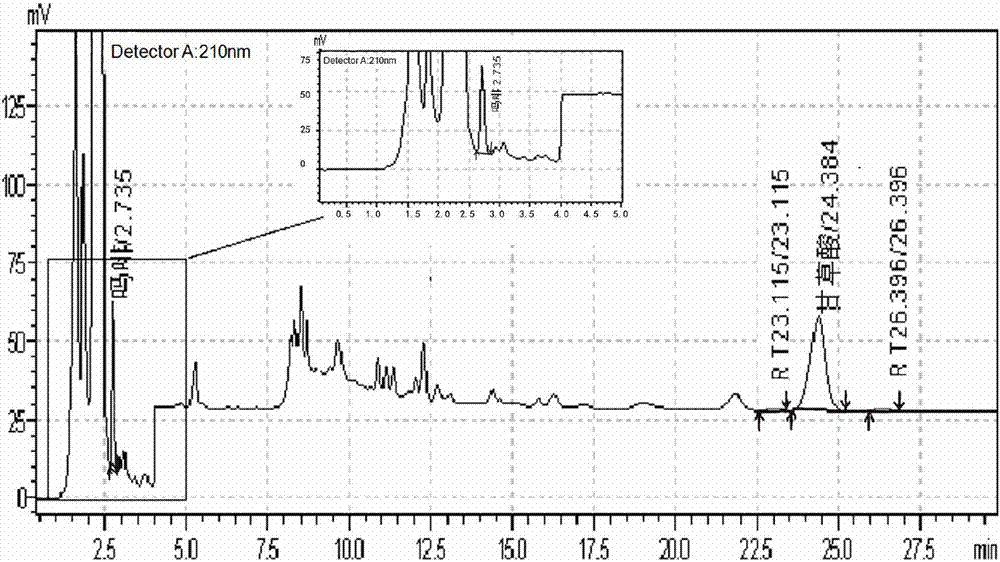
[0104] The selection of the composition ratio in the mobile phase during concentration and gradient elution of embodiment two
[0105] Other conditions are the same as in Example 1, using mobile phase phosphate buffer and acetonitrile concentration, gradient elution, and variable wavelength method;
[0106] 0-4 minutes after the start of elution, phosphate buffer 86%, acetonitrile 14%, wavelength 210nm; 4-4.01 minutes after the start of elution, phosphate buffer 86%→73%, acetonitrile 14%→27%, wavelength 250nm; 4.01-28 minutes after the start of elution, phosphate buffer 73%, acetonitrile 27%, wavelength 250nm; 28-28.01 minutes after the start of elution, phosphate buffer 73%→50%, acetonitrile 27%→50% , wavelength 250nm; 28.01-47 minutes after the start of elution, phosphate buffer 50%, acetonitrile 50%, wavelength 250nm; 47-47.01 minutes after the start of elution, phosphate buffer 50% → 86%, acetonitrile 50% → 14%, wavelength 210nm; 47.01-65 minutes after the start of elutio...
Embodiment 3
[0108] The selection of embodiment three mobile phase pH value
[0109] Other conditions are the same as in Example 1, using phosphate buffer and acetonitrile as the mobile phase. Phosphate buffer solution: Take 6.8g of potassium dihydrogen phosphate, dissolve it in an appropriate amount of water, add 1.1g of sodium heptanesulfonate and 2ml of triethylamine, adjust the pH to 5.1 with 20% phosphoric acid solution, and dilute to 1000ml with water.
[0110] The chromatographic peak of glycyrrhizic acid in the test solution is interfered by adjacent impurity peaks, so it cannot be detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com