Self-repaired organic silicon modified polyurethane elastomer and preparation method thereof
A polyurethane elastomer and silicone technology, applied in the field of polyurethane elastomer, can solve the problems of lack of mechanical properties, heat resistance, weather resistance, etc., and achieve the effect of high-efficiency self-healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In the preparation of polyurethane prepolymer and polyurethane solution, the solvent used when carrying out the solution reaction can be selected from toluene, tetrahydrofuran, acetone, chloroform, dimethyl sulfoxide, N,N-dimethylformamide (DMF ) and one or more of N,N-dimethylacetamide.
[0042]In the preparation of the polyurethane prepolymer, preferably, the diisocyanate group-containing monomer and the active group-terminated polydimethylsiloxane are reacted in solution at 50-100° C. for 2-5 hours to prepare the polyurethane prepolymer. In the preparation of the polyurethane solution, it is preferred to continue to react the obtained polyurethane prepolymer with the disulfide bond-containing monomer in the solution at 50-100° C. for 3-9 hours to prepare the final product Polyurethane copolymer solution.
[0043] In the step of drying the prepared polyurethane solution, the obtained polyurethane solution is cast and dried to prepare a polyurethane elastomer. Speci...
preparation Embodiment 1
[0051] Take 0.336g (2mmol) of 1,6-hexamethylene diisocyanate dissolved in acetone and pour it into a three-necked flask, then take 3g (1mmol) of amino-terminated polydimethylsiloxane dissolved in acetone, drop slowly Add it to the three-necked flask, at 60°C, under stirring at 300rpm, vacuumize and pass through nitrogen to react for 3h to obtain the isocyanate group-terminated prepolymer; then take 0.154g (1mmol) 2,2'-dithiodiethanol , mixed evenly with acetone and then added to the prepolymer to react for 6 hours to obtain a polyurethane copolymer solution.
[0052] After the reaction, the solution was transferred to a polytetrafluoroethylene mold, and dried in an oven at 90° C. for 12 hours to obtain polyurethane elastomer PU-1.
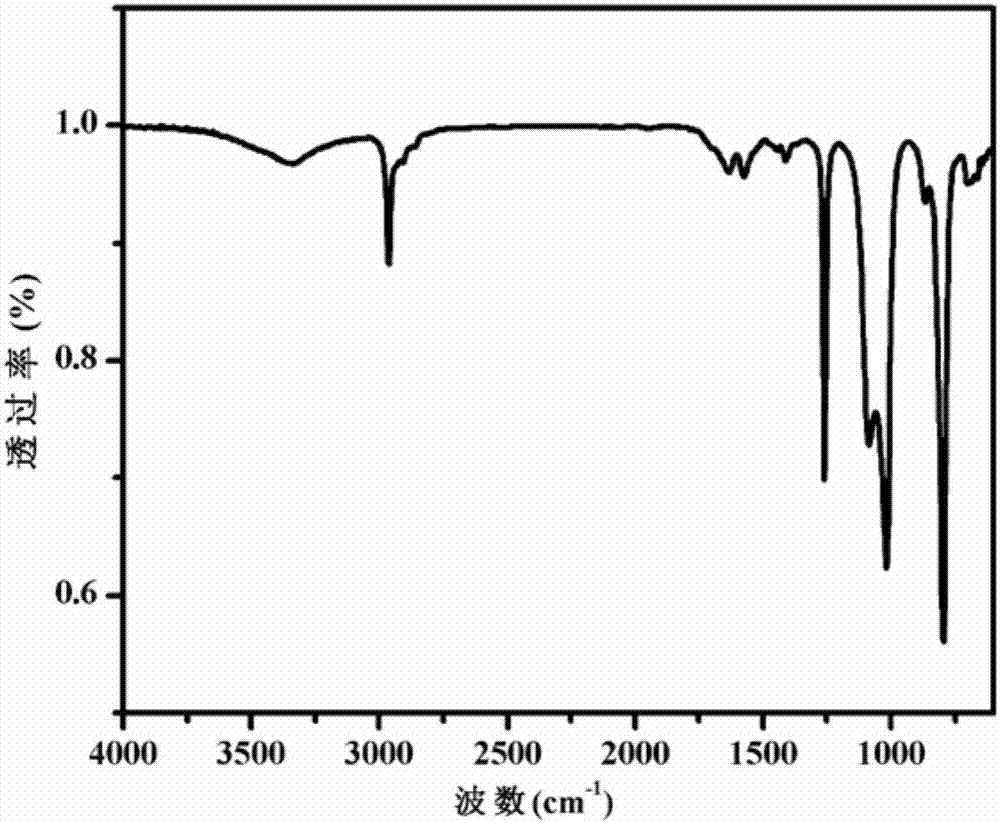
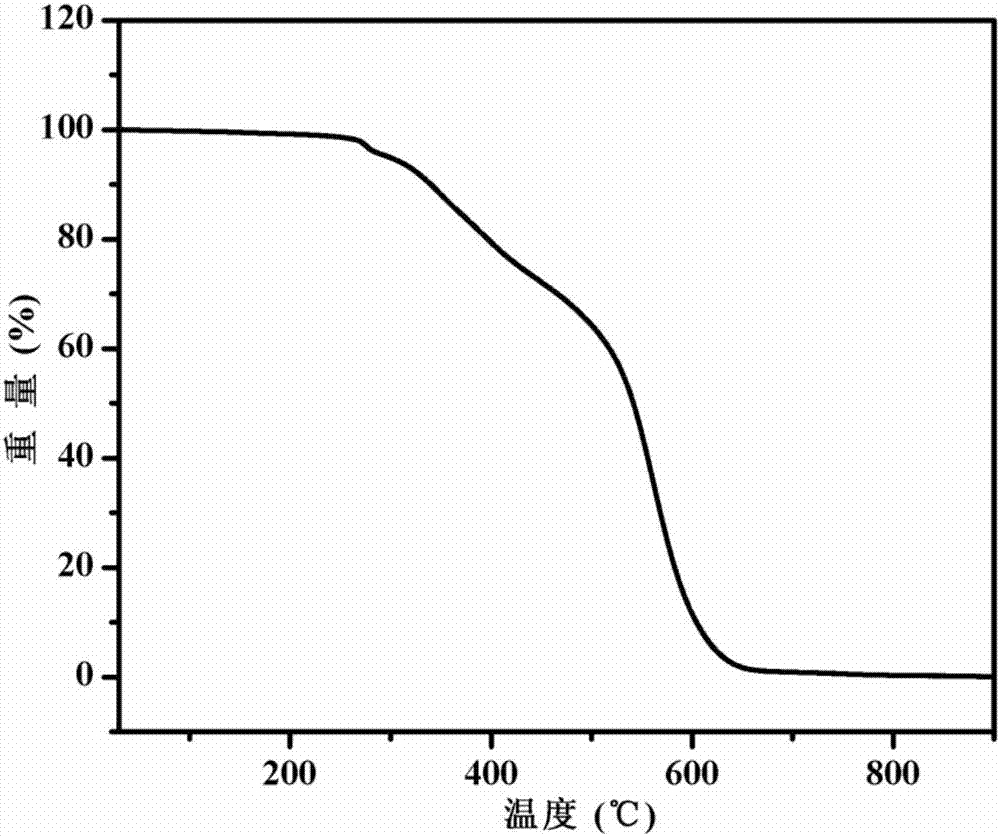
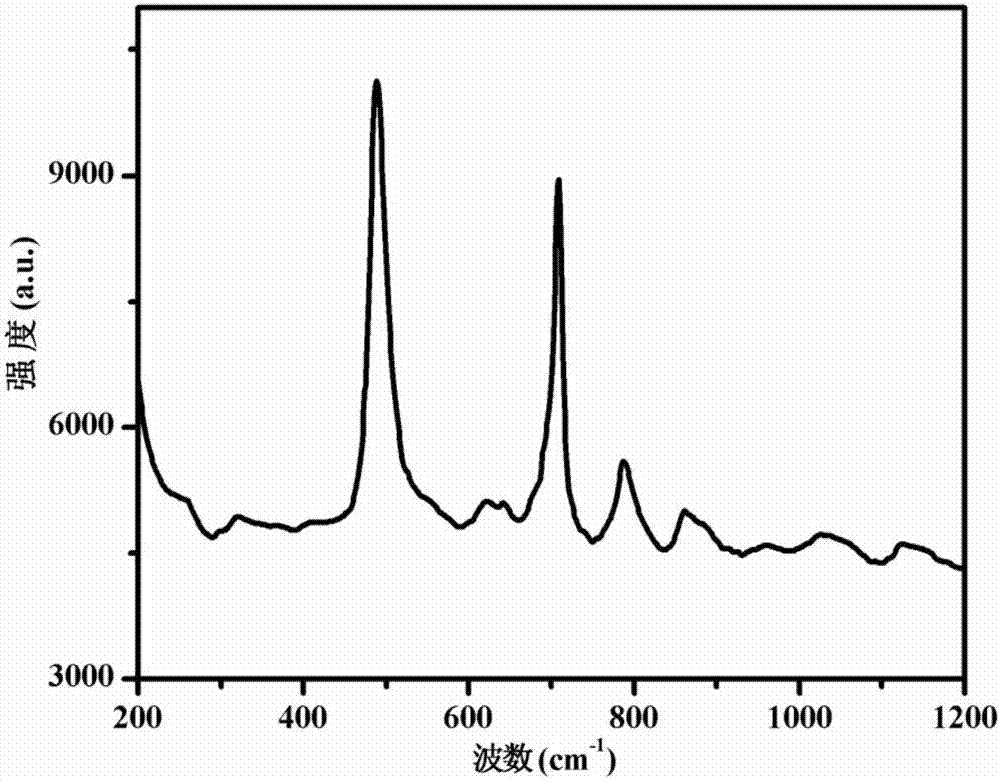
[0053] Figure 1-3 The infrared spectrogram, thermogravimetric analysis (TGA) chart and Raman spectrogram of the obtained PU-1 polyurethane elastomer are shown respectively. The infrared spectrum of polyurethane elastomer PU-1 is tested by Bruker...
preparation Embodiment 2
[0055] Take 0.252g (1.5mmol) of 1,6-hexamethylene diisocyanate and dissolve it in acetone and pour it into a three-necked flask, then take 3g (1mmol) of amino-terminated polydimethylsiloxane and dissolve it in acetone, slowly Add it dropwise into a three-necked flask, at 60°C, under stirring at 300rpm, vacuumize and feed nitrogen for 3 hours to obtain an isocyanate-terminated prepolymer; then take 0.077g (0.5mmol) of 2,2'-disulfide Ethanol, mixed evenly with acetone, was added to the prepolymer to react for 6 hours to obtain a polyurethane copolymer solution.
[0056] After the reaction, the solution was transferred to a polytetrafluoroethylene mold, and dried in an oven at 90° C. for 12 hours to obtain polyurethane elastomer PU-2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com