Benzotriazole compound ultraviolet light absorber as well as preparation method and application thereof
A benzotriazole and compound technology, applied in the field of new benzotriazole compounds, can solve the problems of lack of active groups, affecting the UV resistance and durability of fabrics, etc. The effect of migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
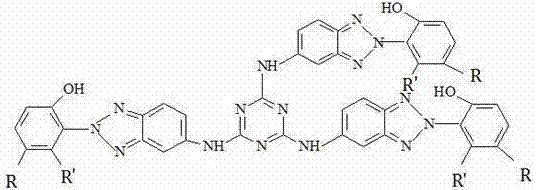
[0025] 100mL there-necked bottle, add 1.84g cyanuric chloride to 30mL water, stir well to form a homogeneous suspension, then slowly add 6.75g of 2-(2-hydroxyphenyl)-5-aminobenzotriazole dropwise into the there-necked bottle For sodium hydroxide solution, the optimal speed of dropping is controlled at 0.5-1ml / min. During the dropping, react at a temperature of 0-5°C for 2 hours, then raise the temperature to 40°C, react for 2 hours, and react at a temperature of 80°C , reacted for 6h, after the reaction was completed, cooled to room temperature, poured the reaction solution into water, a yellow solid precipitated out, stood still, filtered with water, washed with water, washed with ethanol, washed with acetone, and dried to obtain a light yellow solid powder. Wherein, the sodium hydroxide solution can also be potassium hydroxide solution, sodium bicarbonate solution or sodium carbonate solution, and the acid generated by the dropwise equilibrium reaction;
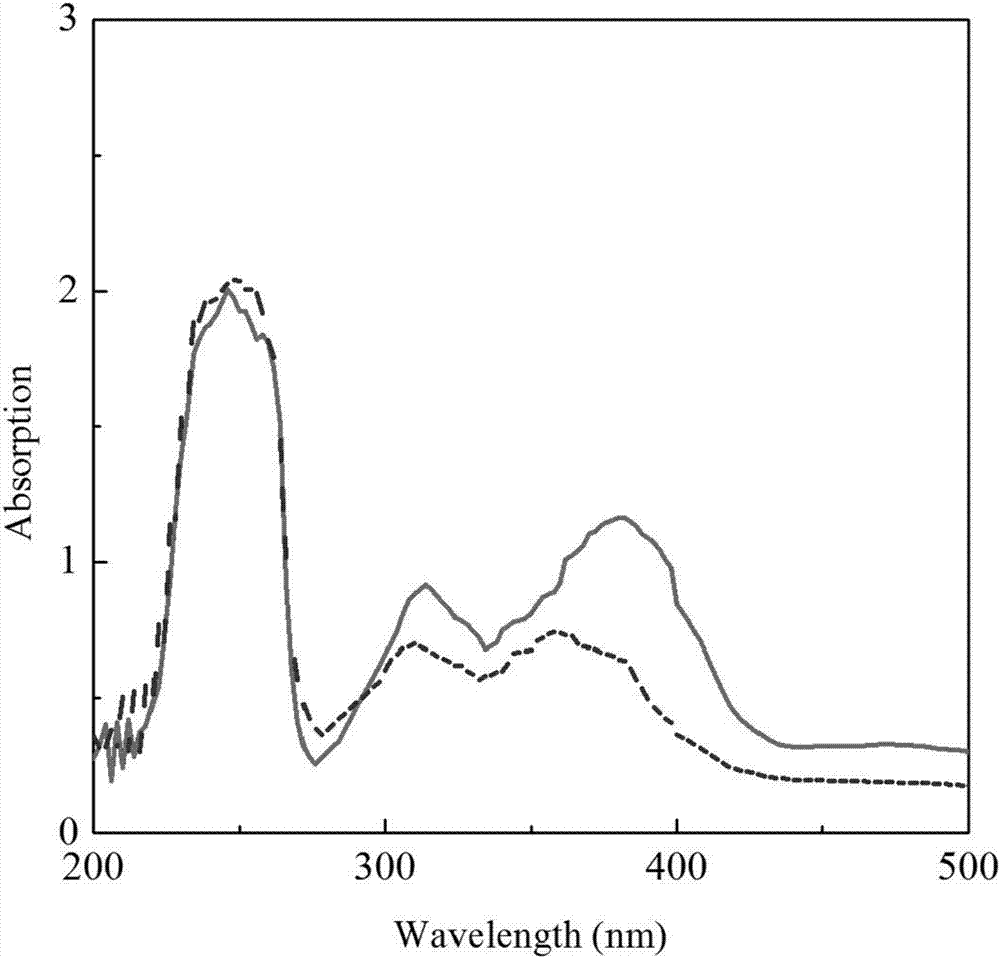
[0026] Anti-ultravi...
Embodiment 2
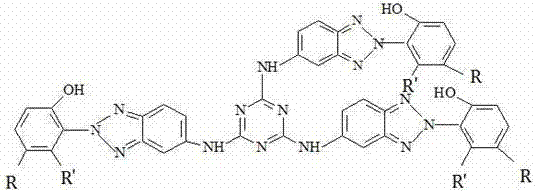
[0029] 100mL there-necked bottle, add 1.84g cyanuric chloride to 30mL water, stir well to form a uniform suspension, then slowly add dropwise sodium hydroxide containing 7.17g 2-(2-hydroxyphenyl)-5-aminobenzotriazole Solution, the best rate of dropping is controlled at 0.5-1ml / min. During the dropping, react at a temperature of 0-5°C for 2 hours, then raise the temperature to 60°C, react for 2 hours, and react at a temperature of 90°C for 5 hours. h. After the reaction, the reaction solution was poured into water, and a yellow solid was precipitated, left to stand, filtered by suction, washed with water, ethanol, and acetone, and dried to obtain a light yellow solid powder. Wherein, the sodium hydroxide solution can also be potassium hydroxide solution, sodium bicarbonate solution or sodium carbonate solution, and the acid generated by the dropwise equilibrium reaction;
[0030] Anti-ultraviolet finishing of polyester fabrics:
[0031] The composition of the finishing agent i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com