Preparation method and application of pH-sensitive hyaluronic acid-doxorubicin nanometer prodrug
A technology of hyaluronic acid and doxorubicin, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problems, to achieve the effect of convenient directional drug delivery, easy storage, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
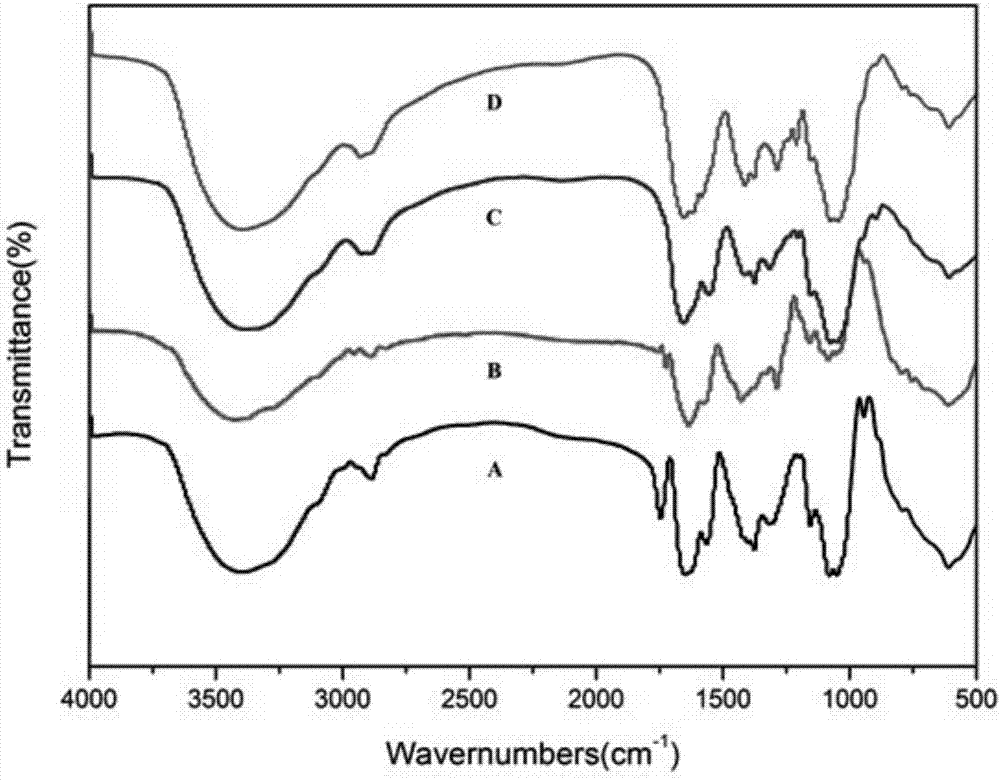
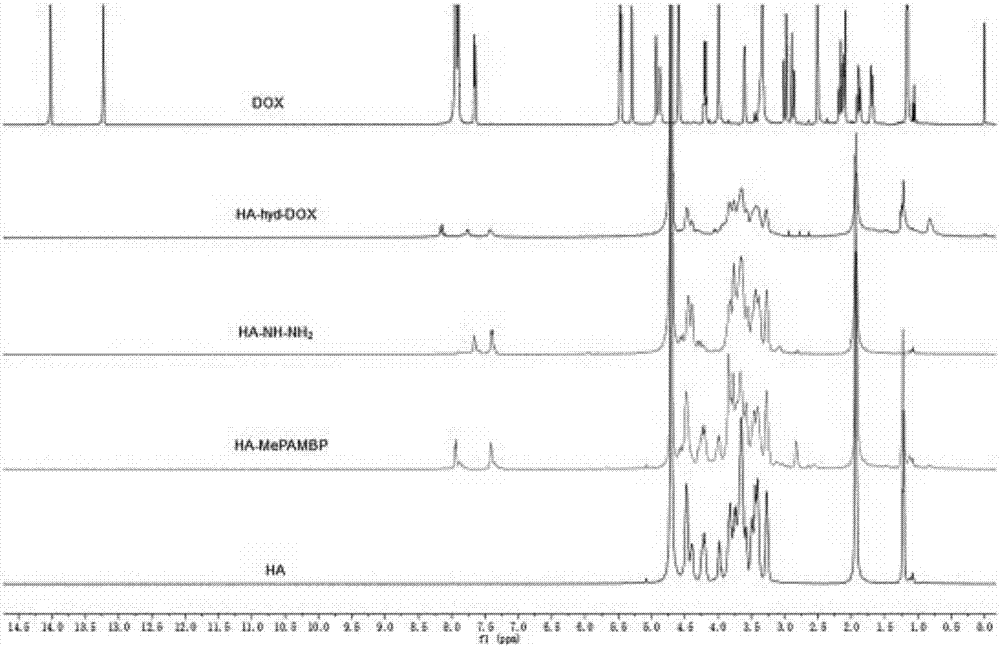
[0034] Dissolve 400mg of hyaluronic acid (HA, molecular weight 5300Da) in 30mL of formamide, and add 256mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) while magnetic stirring HCl), after reacting at room temperature for 30 min, 146 mg of N-hydroxysuccinimide (NHS) was added, and the reaction was continued for 2 h. At the same time, 255 mg of methyl 4-aminomethylbenzoate was dissolved in 15 mL of formamide, and after dissolution, it was added dropwise into the hyaluronic acid activation system, and reacted with magnetic stirring for 24 hours. After the reaction, the reaction mixture was dialyzed with distilled water (MWCO, 3500) for 3 days, and freeze-dried to obtain the product HA-Ester.
[0035] Weigh 300 mg of lyophilized HA-Ester and dissolve it in 15 mL of formamide, then add 1.5 mL of hydrazine hydrate (NH 2 -NH 2 ·H 2 O, mass fraction 85%), reflux reaction at 60° C. for 16 h under nitrogen protection condition. After removing most of the solv...
Embodiment 2
[0039] Dissolve 400mg of hyaluronic acid (HA, molecular weight: 9800Da) in 30mL of formamide, and add 256mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) while magnetically stirring HCl), after reacting at room temperature for 30 min, 146 mg of N-hydroxysuccinimide (NHS) was added, and the reaction was continued for 2 h. At the same time, 255 mg of methyl 4-aminomethylbenzoate was dissolved in 15 mL of formamide, and after dissolution, it was added dropwise into the hyaluronic acid activation system, and reacted with magnetic stirring for 24 hours. After the reaction, the reaction mixture was dialyzed with distilled water (MWCO, 3500) for 3 days, and freeze-dried to obtain the product HA-Ester.
[0040] Weigh 300 mg of lyophilized HA-Ester and dissolve it in 15 mL of formamide, then add 1.5 mL of hydrazine hydrate (NH 2 -NH 2 ·H 2 O, mass fraction 85%), reflux reaction at 60° C. for 16 h under nitrogen protection condition. After removing most of the...
Embodiment 3
[0044] Dissolve 400mg of hyaluronic acid (HA, molecular weight 37000Da) in 30mL of formamide, and add 256mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) while magnetically stirring HCl), after reacting at room temperature for 30 min, 146 mg of N-hydroxysuccinimide (NHS) was added, and the reaction was continued for 2 h. At the same time, 255 mg of methyl 4-aminomethylbenzoate was dissolved in 15 mL of formamide, and after dissolution, it was added dropwise into the hyaluronic acid activation system, and reacted with magnetic stirring for 24 hours. After the reaction, the reaction mixture was dialyzed with distilled water (MWCO, 3500) for 3 days, and freeze-dried to obtain the product HA-Ester.
[0045] Weigh 300 mg of lyophilized HA-Ester and dissolve it in 15 mL of formamide, then add 1.5 mL of hydrazine hydrate (NH 2 -NH 2 ·H 2 O, mass fraction 85%), reflux reaction at 60° C. for 16 h under nitrogen protection condition. After removing most of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
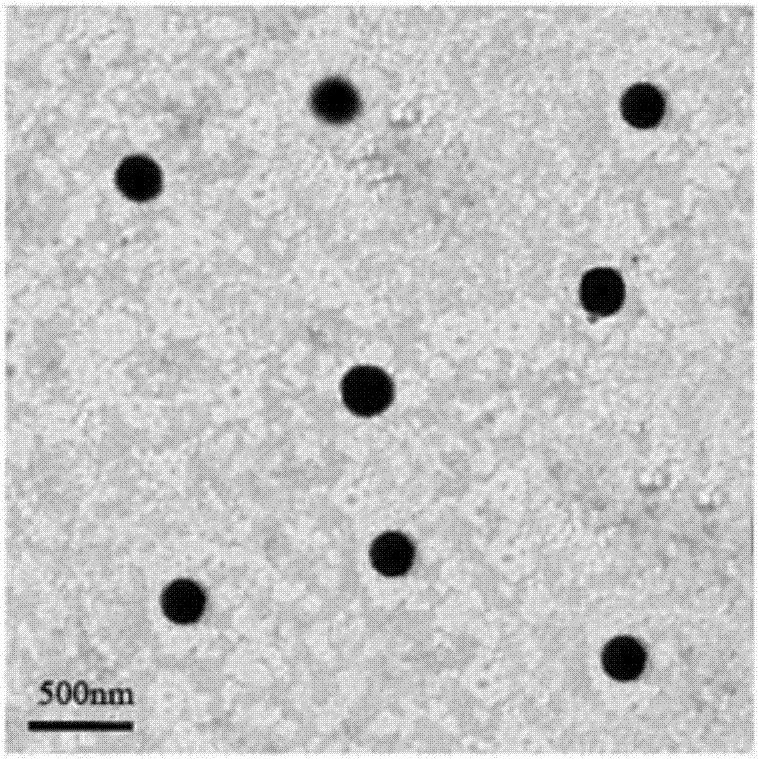
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com