Method used for synthesizing alpha-ketoamides via micro flow field technology continuous flow
A technology of flow synthesis and microfluidic field, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc. Response, shortened response time, effect of short contact time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
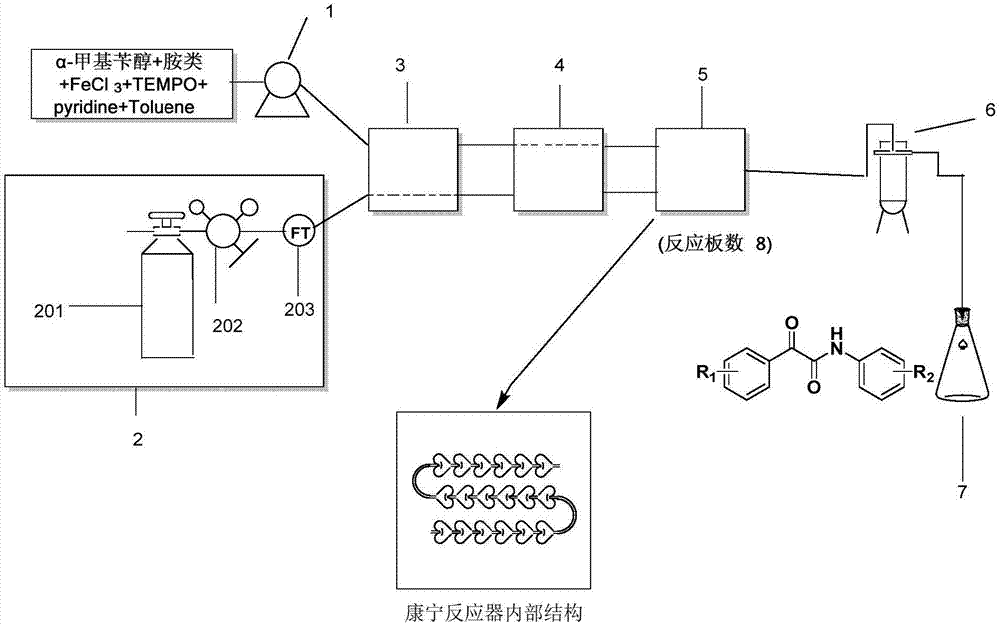
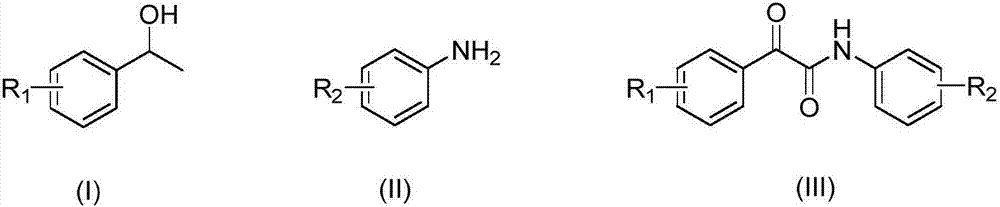
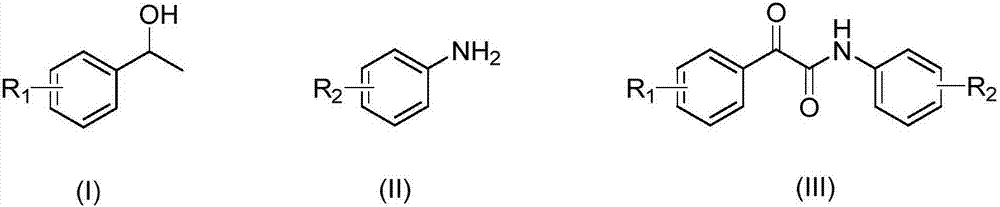
[0026] Weigh 2.82g, 0.02mol specification of 2,2,6,6,-tetramethylpiperidine oxide fully dissolved in 50ml of toluene solvent, then weigh 12.21g, 0.1mol specification of α-methylbenzyl alcohol, 11.81 g, 0.1 mol of p-aminobenzonitrile, 15.82 g, 0.2 mol of pyridine in the above solvent, add toluene solvent to make the total volume to 200 ml. Add 1.62 g, 0.01 mol ferric chloride powder to the above fixed liquid, and stir it into a uniform heterogeneous state with a stirrer. Feed nitrogen back pressure to the Corning reactor to 10bar, switch to oxygen, and pump the heterogeneous solution into the reactor at the same time. Keep the reaction temperature at 80°C, the heterogeneous solution flow rate is 10ml / min, the gas flow rate is 0.5L / min, and the reaction time is about 1.1min, that is, 1.1min after the reaction starts, the reaction solution is collected in the collection bottle. According to high-performance liquid chromatography analysis, in this reaction, the conversion rate of...
Embodiment 2
[0028] Weigh 2.82g, 0.02mol specification of 2,2,6,6,-tetramethylpiperidine oxide fully dissolved in 50ml of toluene solvent, then weigh 12.21g, 0.1mol specification of α-methylbenzyl alcohol, 23.62g, 0.2mol of p-aminobenzonitrile, 15.82g, 0.2mol of pyridine in the above solvent, and add toluene solvent to make the total volume to 200ml. Add 1.62 g, 0.01 mol ferric chloride powder to the above fixed liquid, and stir it into a uniform heterogeneous state with a stirrer. Feed nitrogen back pressure to the Corning reactor to 10bar, switch to oxygen, and pump the heterogeneous solution into the reactor at the same time. Keep the reaction temperature at 80°C, the heterogeneous solution flow rate is 10ml / min, the gas flow rate is 0.5L / min, and the reaction time is about 1.1min, that is, 1.1min after the reaction starts, the reaction solution is collected in the collection bottle. Through high-performance liquid chromatography analysis, in this reaction, the conversion rate of the α...
Embodiment 3
[0030] Weigh 2.82g, 0.02mol specification of 2,2,6,6,-tetramethylpiperidine oxide fully dissolved in 50ml of toluene solvent, then weigh 12.21g, 0.1mol specification of α-methylbenzyl alcohol, 35.44 g, 0.3 mol of p-aminobenzonitrile, 15.82 g, 0.2 mol of pyridine in the above solvent, and add toluene solvent to make the total volume to 200 ml. Add 1.62 g, 0.01 mol ferric chloride powder to the above fixed liquid, and stir it into a uniform heterogeneous state with a stirrer. Feed nitrogen back pressure to the Corning reactor to 10bar, switch to oxygen, and pump the heterogeneous solution into the reactor at the same time. Keep the reaction temperature at 80°C, the heterogeneous solution flow rate is 10ml / min, the gas flow rate is 0.5L / min, and the reaction time is about 1.1min, that is, 1.1min after the reaction starts, the reaction solution is collected in the collection bottle. According to high-performance liquid chromatography analysis, in this reaction, the conversion rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com