A method for improving the luminous efficiency of europium complexes
A technology of complexes and ligands, which can be applied to luminescent materials, compounds containing Group 3/13 elements of the periodic table, and Group 3/13 organic compounds without C-metal bonds, etc., which can solve problems such as low metal ion efficiency , to achieve the effect of simple fluorescence efficiency, simple and fast synthesis method, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Rare earth-organic complexes [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] . 2DMF and [Eu 2 (qc) 6 (H 2 O) 6 ]. 3H 2 Synthesis of O
[0020] Weigh the ligand quinoline-2-carboxylic acid Hqc 40 mg, europium acetate Eu(CH 3 COO) 3 . 6H 2 O 180 mg, sodium ethoxide CH 3 CH 2 ONa 41 mg in a 50 ml Erlenmeyer flask, add 20 ml of ethanol C to this mixture 2 h 5 Oh. After ultrasonication for 10 minutes and uniform mixing, the Erlenmeyer flask containing the reaction substance was placed on a magnetic stirrer and stirred at room temperature for 24 hours. Filter the above mixture, and the filtrate obtained is volatilized at room temperature to obtain colorless rhombohedral crystals [Eu 2 (qc) 6 (H 2 O) 6 ] . 3H 2 O. Dilute the filter residue obtained by the above filtration with 10 ml N,N-dimethylformamide DMF and 3 ml water H 2 O is heated and dissolved, and a colorless cube-shaped single crystal product [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] ...
Embodiment 2
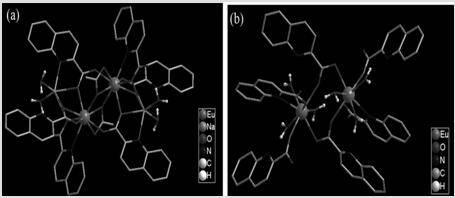
[0022] Rare earth-organic complexes [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] . 2DMF and [Eu 2 (qc) 6 (H 2 O) 6 ] . 3H 2 The structure and difference of O
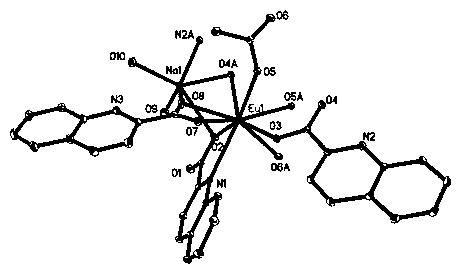
[0023] Complex [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] . The single crystal structure of 2DMF was obtained by the Saturn724+ CCD of Japan Rigaku X - Measured by X-ray single crystal diffractometer. The crystal structure was analyzed and determined using the program Shexl 2014. Complex [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] . 2DMF crystallized in P 2 1 / c space group, monoclinic crystal system. There is one europium ion Eu in the asymmetric unit 3+ , three deprotonated quinoline-2-carboxylic acid ligands qc - , a sodium ion Na + , both with Na + Ionically coordinated water molecule H 2 O, a coordinated acetate with ion CH 3 COO - and a molecule of N,N-dimethylformamide DMF in a lattice. Eu 3+ The ions have a nine-coordinated geometry. The nine coordinating atoms are four qc...
Embodiment 3
[0025] Rare earth-organic complexes [Na 2 Eu 2 (qc) 6 (CH 3 COO) 2 (H 2 O) 4 ] . The purity and stability of 2DMF
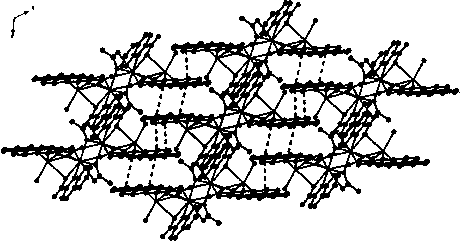
[0026] The purity of the complex was determined using a Japanese Rigaku DMAX-2500 copper rake X - Measured by X-ray powder diffractometer. The simulated powder diffraction pattern is consistent with the reagent diffraction pattern of the sample, which proves the high purity of the prepared complex. In addition, the thermal stability of the complex was measured and characterized by the STA-449C thermogravimetric analyzer of the German NETZSCH company. The complex has certain thermal stability below 300°C and can maintain the stability of the molecular skeleton.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com