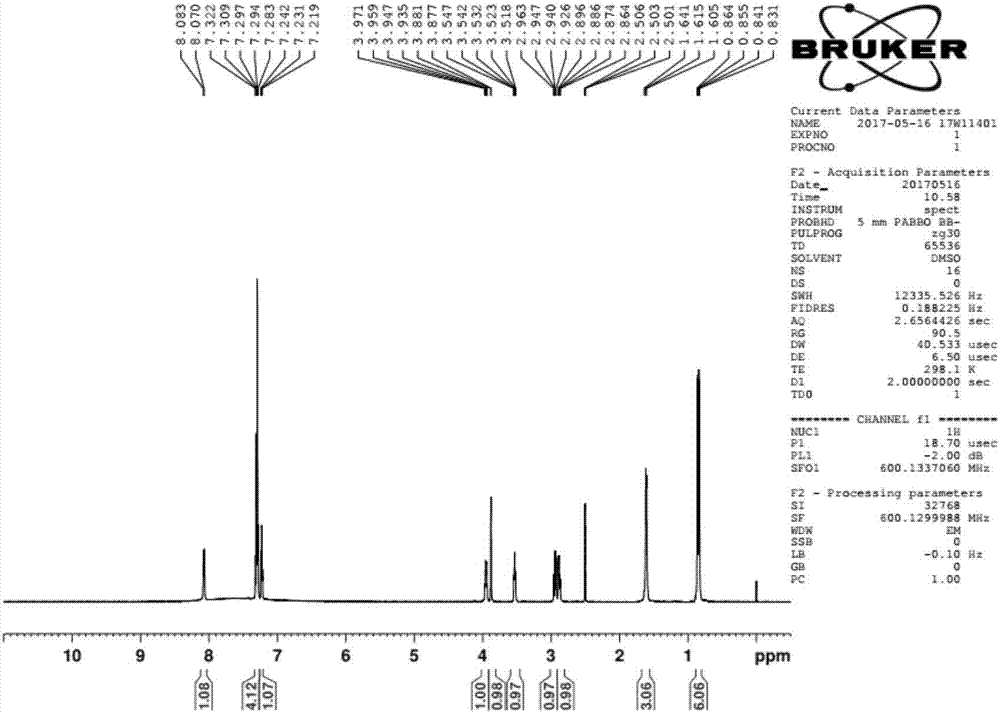
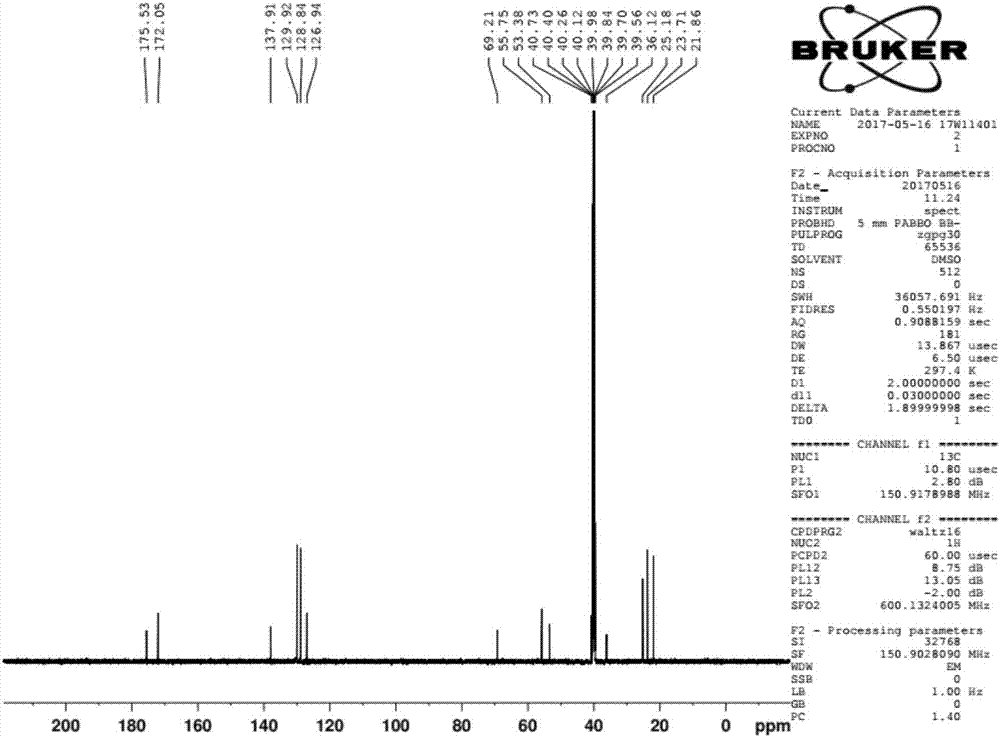
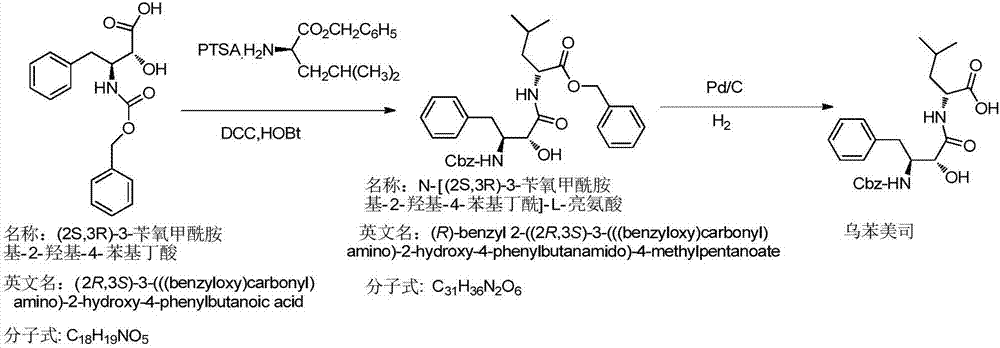
Preparation method of ubenimex
A technology of ubimethoxine and phenylbutyryl, applied in the field of preparation of ubimethoxine, can solve the problems of large processing burden, easy residue, organic solvent residue and the like, and achieves the effects of cost saving and solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation method of Ubenimex is as follows:
[0038] 1) Add L-leucine benzyl ester p-toluenesulfonate, HOBt, and 10 times (V / m) ethyl acetate into the reaction kettle in sequence, after the feeding is completed, keep stirring and cooling down, and slowly add the ethyl acetate solution of DCC, Adding process system temperature between 0-5 ℃. After feeding, the system was reacted at 15-20°C for 4 hours, followed by TLC at the end of the reaction (ethyl acetate: petroleum ether = 2:1, UV 254nm), and the activated ester solution of L-leucine benzyl ester p-toluenesulfonate was obtained ;
[0039] 2) Add (2S,3R)-3-benzyloxycarboxamido-2-hydroxy-4-phenylbutyric acid, 10 times (V / m) 10% sodium bicarbonate solution into the reaction kettle in sequence, and stir Until it is completely dissolved, cool down to 10-15°C, add the activated ester solution of L-leucine benzyl p-toluenesulfonate dropwise under rapid stirring, and react at 15-20°C for about 4 hours after the drop...
Embodiment 2
[0045] The preparation method of Ubenimex is as follows:
[0046] 1) Add L-leucine benzyl ester p-toluenesulfonate, HOBt, and 10 times (V / m) ethyl acetate into the reaction kettle in sequence, after the feeding is completed, keep stirring and cooling down, and slowly add the ethyl acetate solution of DCC, Adding process system temperature between 0-5 ℃. After feeding, the system was reacted at 15-20°C for 5 hours, followed by TLC at the end of the reaction (ethyl acetate: petroleum ether = 2:1, UV 254nm), and the activated ester solution of L-leucine benzyl ester p-toluenesulfonate was obtained ;
[0047] 2) Add (2S,3R)-3-benzyloxycarboxamido-2-hydroxy-4-phenylbutyric acid, 10 times (V / m) 20% sodium bicarbonate solution into the reaction kettle in sequence, and stir Until it is completely dissolved, cool down to 10-15°C, add dropwise the activated ester solution of L-leucine benzyl p-toluenesulfonate under rapid stirring, and react at 15-20°C for about 5 hours after the addi...
Embodiment 3
[0053] The preparation method of Ubenimex is as follows:
[0054] 1) Add L-leucine benzyl ester p-toluenesulfonate, HOBt, and 10 times (V / m) ethyl acetate into the reaction kettle in sequence, after the feeding is completed, keep stirring and cooling down, and slowly add the ethyl acetate solution of DCC, Adding process system temperature between 0-5 ℃. After feeding, the system was reacted at 15-20°C for 4 hours, followed by TLC at the end of the reaction (ethyl acetate: petroleum ether = 2:1, UV 254nm), and the activated ester solution of L-leucine benzyl ester p-toluenesulfonate was obtained ;
[0055] 2) Add (2S,3R)-3-benzyloxycarboxamido-2-hydroxy-4-phenylbutyric acid, 10 times (V / m) 20% sodium bicarbonate solution into the reaction kettle in sequence, and stir Until it is completely dissolved, cool down to 10-15°C, add the activated ester solution of L-leucine benzyl p-toluenesulfonate dropwise under rapid stirring, and react at 15-20°C for about 4 hours after the drop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com