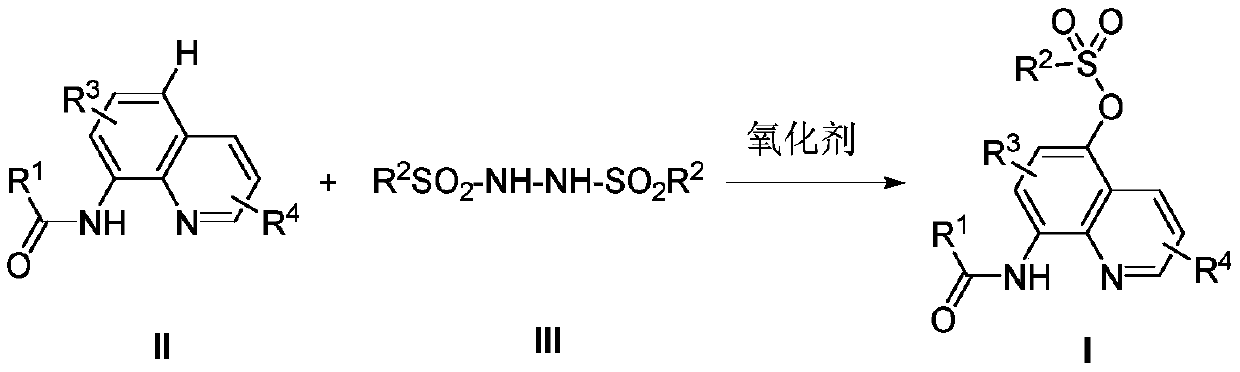
A kind of preparation method of 5-sulfonyloxy-8-amidoquinoline derivative
A technology of sulfonyloxy, quinoline, applied in the preparation of 5-sulfonyloxy-8-amidoquinoline derivatives, in the field of preparing 5-sulfonyloxy-8-acetamidoquinoline, capable of Solve the problems of expensive price, many reaction steps, and low yield, and achieve the effect of wide substrate adaptability, low-cost raw materials, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
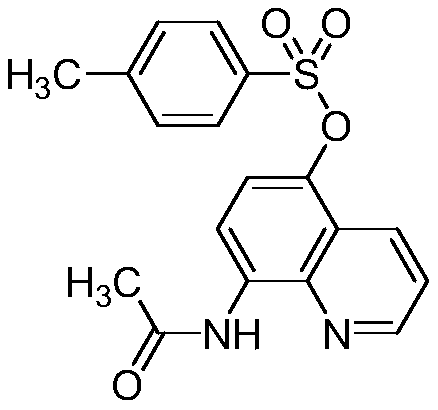
[0015] Preparation of 5-(4-methylbenzenesulfonyloxy)-8-acetamidoquinoline (I-1)
[0016]
[0017] Add tetrahydrofuran (100mL), 8-acetamidoquinoline (II-1) (1.86g, 0.01mol), N,N'-bis(4-methylbenzenesulfonyl)hydrazine (III- 1) (3.70g, 0.011mol) and iodobenzene trifluoroacetate (14.2g, 0.033mol), stirred at room temperature for 1 hour, filtered, washed the filter cake with dichloromethane, removed the solvent under reduced pressure to obtain crude product, column chromatography ( Petroleum ether / ethyl acetate as eluent, gradient elution) to obtain 2.78 g of white solid, yield 78%, m.p.163-167°C. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 9.78 (s, 1H), 8.85 (d, J = 2.8Hz, 1H), 8.65 (d, J = 8.6Hz, 1H), 8.44 (d, J = 8.4Hz, 1H), 7.79 (d,J=8.1Hz,2H),7.53(dd,J=8.4,4.2Hz,1H),7.36(d,J=7.9Hz,2H),7.01(d,J=8.6Hz,1H),2.49 (s,3H),2.38(s,3H); 13 C NMR (75MHz, CDCl 3 )δ (ppm): 168.27, 148.29, 145.29, 138.95, 133.31, 131.67, 130.84, 129.44, 128.14, 126.77, 122.41, 121.60, 119.31, 114.62, 24.52, 2...
Embodiment 2
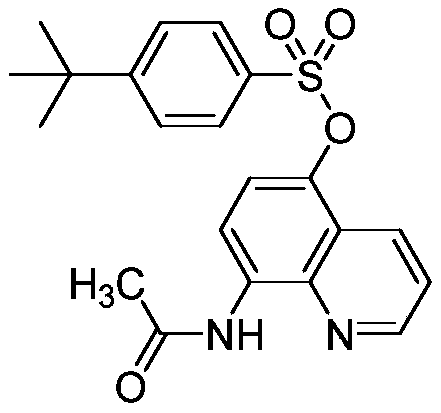
[0019] Preparation of 5-(4-tert-butylbenzenesulfonyloxy)-8-acetamidoquinoline (I-2)
[0020]
[0021] Add tetrahydrofuran (100mL), II-1 (1.86, 0.01mol), N,N'-di(4-tert-butylbenzenesulfonyl)hydrazine (III-2) (4.66g, 0.011mmol) into a 250mL four-neck flask and iodobenzene trifluoroacetate (14.2g, 0.033mmol), stirred and reacted at room temperature for 1 hour, filtered, washed the filter cake with dichloromethane, and removed the solvent under reduced pressure to obtain a crude product. Column chromatography (petroleum ether / ethyl acetate was eluting reagent, gradient elution) to obtain white solid 2.71g, yield 68%, m.p.142-145 ℃. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 9.74 (s, 1H), 8.81 (s, 1H), 8.66 (d, J = 8.4Hz, 1H), 8.33 (d, J = 8.5Hz, 1H), 7.81 (d, J = 7.1Hz, 2H), 7.55(d, J=7.1Hz, 2H), 7.47(s, 1H), 7.08(d, J=7.5Hz, 1H), 2.37(s, 3H), 1.36(s, 9H) ; 13 C NMR (75MHz, CDCl 3 )δ (ppm): 168.26, 158.30, 148.20, 138.91, 137.83, 133.30, 131.59, 130.74, 127.95, 125.82, 122.38, 121....
Embodiment 3
[0023] Preparation of 5-Benzenesulfonyloxy-8-acetamidoquinoline (I-3)
[0024]
[0025] Use II-1 (1.86g, 0.01mol), N,N'-diphenylsulfonylhydrazine (III-3) (3.43g, 0.011mmol) and iodobenzene trifluoroacetate (14.2g, 0.033mmol) as raw materials. The operation was the same as in Example 1 to obtain 2.46 g of a white solid with a yield of 72%, m.p.165-168°C. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 9.77 (s, 1H), 8.85 (d, J = 4.3Hz, 1H), 8.67 (d, J = 8.6Hz, 1H), 8.39 (d, J = 8.4Hz, 1H), 7.92 (d, J=7.3Hz, 2H), 7.72(t, J=7.4Hz, 1H), 7.60–7.48(m, 3H), 7.04(d, J=8.6Hz, 1H), 2.38(s, 3H) ; 13 C NMR (75MHz, CDCl 3 )δ (ppm): 168.33, 148.33, 138.34, 137.61, 134.49, 134.07, 130.67, 128.86, 128.10, 122.29, 121.67, 119.40, 119.30, 114.59, 24.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com