Method for efficiently and rapidly preparing and separating three kinds of ginsenoside isomers Rg6, Z type and E type F4
A technology of ginsenosides and isomers, applied in chemical instruments and methods, glycoside steroids, steroids, etc., can solve the problems of difficulty in obtaining standard products, limited activity, low yield, etc., and is convenient for pharmacodynamics research. , The effect of high utilization rate of raw materials and high separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
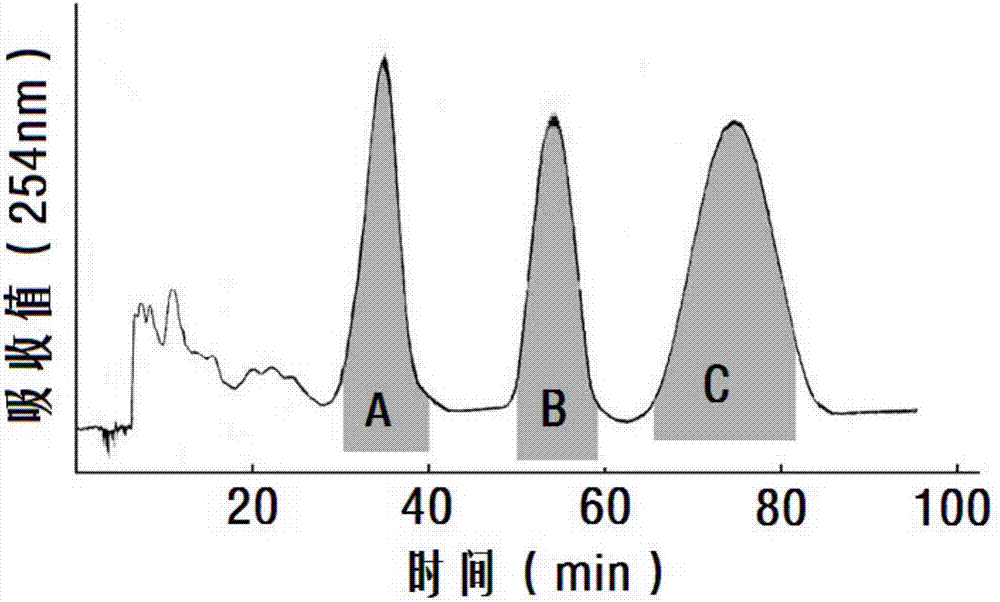
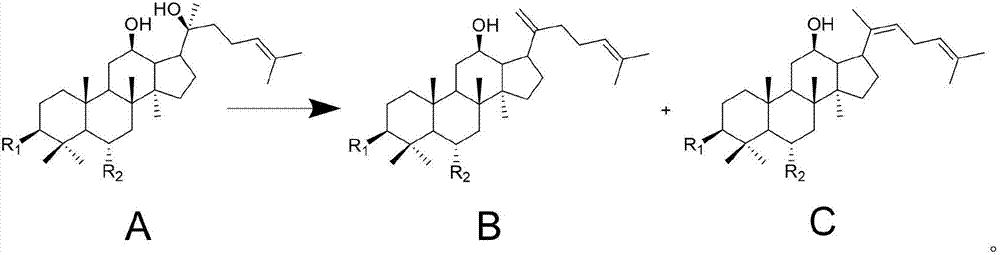
[0032] Example 1 Preparation of Ginsenoside Isomer Rg6, Z Type and E Type F4 Mixture and Separation of Mixture
[0033] How to prepare the mixture:
[0034] Take 500mg ginsenoside Rg2 (self-made, purity greater than 95%), dissolve with 200mL methanol aqueous solution (methanol concentration is 50% by volume) containing formic acid, the volume of formic acid added is 0.05% of the volume of methanol water. After dissolving, 50 mg of lead dioxide particles were added to the reaction system, and the reaction was refluxed at 85° C. for 2.5 hours. After the reaction, it is naturally cooled to room temperature, filtered to remove lead dioxide particles, the filtrate is concentrated into an aqueous solution substantially free of methanol, and freeze-dried to obtain a freeze-dried powder.
[0035] High-speed countercurrent separation method for mixtures:
[0036] Prepare ethyl acetate-ethanol-water-acetic acid (volume ratio 4:1:5:0.08) solvent system 12 hours before separation. The s...
Embodiment 2
[0043] Example 2 Preparation of ginsenoside isomer Rg6, Z-type and E-type F4 mixture and separation of the mixture
[0044] How to prepare the mixture:
[0045]Get 500mg ginsenoside Rg2 (self-made, purity greater than 95%), dissolve with 200mL ethanol aqueous solution (volume percent concentration of ethanol is 50%) containing acetic acid, and the volume of acetic acid added is 0.05% of the volume of ethanol water. After dissolving, 50 mg of lead dioxide particles were added to the reaction system, and the reaction was refluxed at 85° C. for 2.5 hours. After the reaction, cool naturally to room temperature, filter to remove lead dioxide particles, concentrate the filtrate into an aqueous solution substantially free of ethanol, and freeze-dry to obtain freeze-dried powder.
[0046] High-speed countercurrent separation method for mixtures:
[0047] Prepare ethyl acetate-ethanol-water-acetic acid (volume ratio 4:1:5:0.08) solvent system 12 hours before separation. The specific ...
Embodiment 3
[0051] Example 3 Preparation of ginsenoside isomer Rg6, Z-type and E-type F4 mixture and separation of the mixture
[0052] How to prepare the mixture:
[0053] Take 500mg of ginsenoside Rg2 (self-made, purity greater than 95%), dissolve with 200mL of methanol aqueous solution (40% by volume of methanol) containing formic acid, the volume of formic acid added is 0.06% of the volume of methanol water. After dissolving, 50 mg of lead dioxide particles were added to the reaction system, and the reaction was refluxed at 80° C. for 3 hours. After the reaction, it is naturally cooled to room temperature, filtered to remove lead dioxide particles, the filtrate is concentrated into an aqueous solution substantially free of methanol, and freeze-dried to obtain a freeze-dried powder.
[0054] High-speed countercurrent separation method for mixtures:
[0055] Prepare ethyl acetate-ethanol-water-acetic acid (volume ratio 4:1:5:0.08) solvent system 12 hours before separation. The specifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com