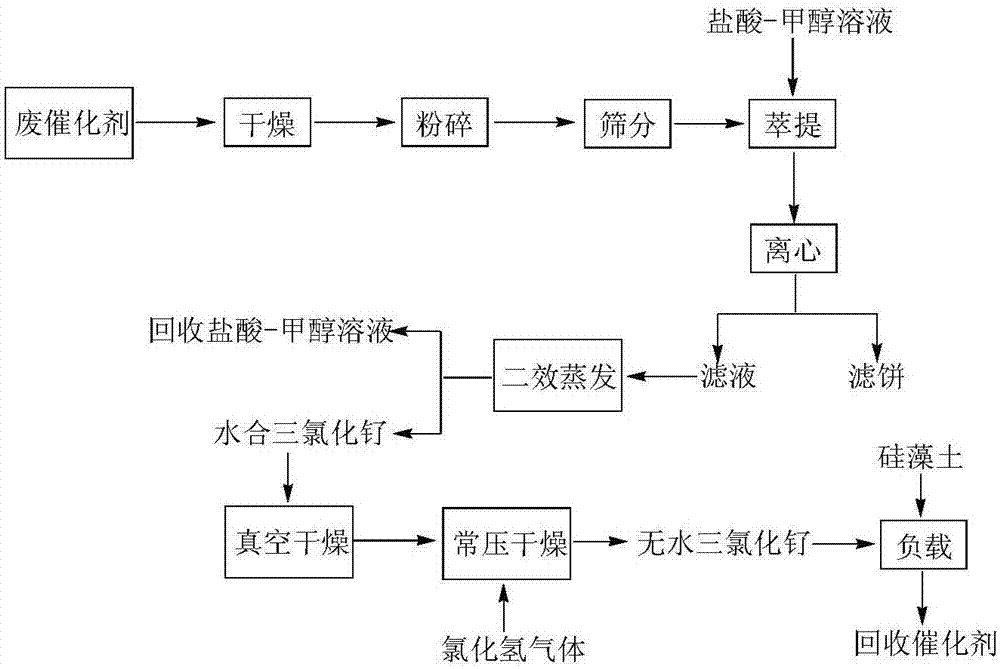
Recovery process of ruthenium trichloride catalyst in trimethylpyruvic acid synthesis work procedure
A technology of ruthenium trichloride catalyst and waste ruthenium trichloride catalyst, which is applied in chemical recovery, preparation of ruthenium/rhodium/palladium/osmium/iridium/platinum compound, inorganic chemistry, etc. Enterprise production and operating costs and other issues to achieve the effect of avoiding energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] With 100 grams of spent ruthenium trichloride catalyst after being dried, pulverized, crossing 50 mesh sieves, add the mixed solution of 1000 grams of hydrochloric acid and methanol (the mass ratio of hydrochloric acid and methanol is 1: 1, wherein the solute mass fraction of hydrochloric acid is 30% ), stirred at 40°C for 5 hours, and centrifuged for solid-liquid separation;
[0023] Above-mentioned centrifugal filtrate is evaporated and recovered hydrochloric acid-methanol mixed solution by two-effect graphite evaporator, and residual ruthenium chloride hydrate is left in the evaporator;
[0024] The above-mentioned ruthenium chloride hydrate was vacuum-dried at 40°C and an absolute pressure of 10 Pa for 5 hours. After the system returned to normal pressure, it was dried at 80°C for 8 hours under the protection of a hydrogen chloride gas flow with a flow rate of 5 cubic meters per hour to recover anhydrous trichloride. Ruthenium.
[0025] Take 1 gram of the recovered...
Embodiment 2
[0035] 100 grams of spent ruthenium trichloride catalyst after being dried, pulverized and crossed 80 mesh sieves was added to the mixed solution of 500 grams of hydrochloric acid and methanol (the mass ratio of hydrochloric acid and methanol is 1:1, wherein the solute mass fraction of hydrochloric acid is 30% ), stirred at 50°C for 4 hours, and centrifuged for solid-liquid separation;
[0036] Above-mentioned centrifugal filtrate is evaporated and recovered hydrochloric acid-methanol mixed solution by two-effect graphite evaporator, and residual ruthenium chloride hydrate is left in the evaporator;
[0037] The above-mentioned ruthenium chloride hydrate was vacuum-dried at 45°C and an absolute pressure of 10Pa for 4 hours. After the system returned to normal pressure, it was dried at 90°C for 6 hours under the protection of a hydrogen chloride gas flow with a flow rate of 8 cubic meters per hour to recover anhydrous trichloride. Ruthenium.
[0038] After compounding 5 grams ...
Embodiment 3
[0042] With 100 grams of spent ruthenium trichloride catalysts after drying, pulverizing and crossing 100 mesh sieves, add a mixed solution of 200 grams of hydrochloric acid and methanol (the mass ratio of hydrochloric acid and methanol is 2:1, wherein the solute mass fraction of hydrochloric acid is 30% ), stirred at 60°C for 2 hours, and centrifuged for solid-liquid separation;
[0043] Above-mentioned centrifugal filtrate is evaporated and recovered hydrochloric acid-methanol mixed solution by two-effect graphite evaporator, and residual ruthenium chloride hydrate is left in the evaporator;
[0044] The above-mentioned ruthenium chloride hydrate was vacuum-dried at 50°C and an absolute pressure of 10Pa for 3 hours. After the system returned to normal pressure, it was dried at 100°C for 5 hours under the protection of a hydrogen chloride gas flow with a flow rate of 10 cubic meters per hour to recover anhydrous trichloride. Ruthenium.
[0045] After compounding 10 grams of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com