Preparation of lanthanide metal-organic framework material and method for visually assaying chiral enantiomers
An organic framework and lanthanide metal technology, applied in the fields of analytical chemistry, metal organic compounds, and material synthesis, can solve the problems of high price, low detection sensitivity, complicated analysis procedures, etc., and achieve low equipment requirements, high fluorescence intensity, and simple process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
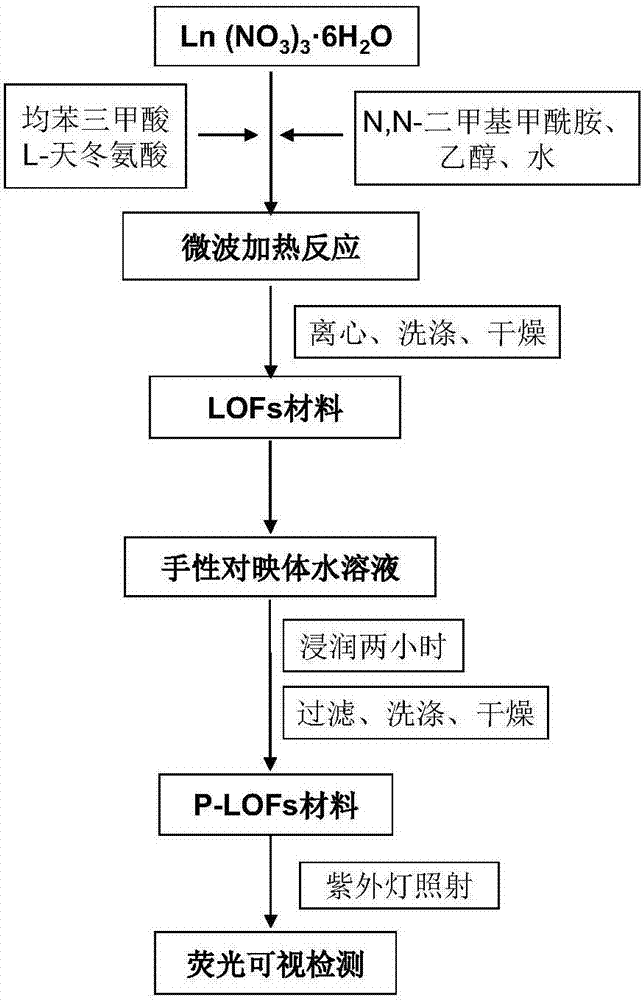
[0027] (1) Weigh a certain amount of lanthanide nitrate hexahydrate (Ln(NO 3 ) 3 ·6H 2 O, Ln=Tb and Eu) and organic ligands (trimesic acid BTC and L-aspartic acid L-Asp), then add a certain amount of N,N-dimethylformamide (DMF), ethanol and water, shake well to dissolve and form a mixed solution. Ln(NO 3 ) 3 ·6H 2 The ratio of O to organic ligand is 1mmol:1mmol; the ratio of Eu to Tb is 1mmol:9.0mmol; the ratio of BTC to L-Asp is 1mmol:1mmol.
[0028] (2) Place the above mixed solution in a quartz microwave reaction tube, heat the reaction by microwave at 100°C for 5min, and cool to room temperature;
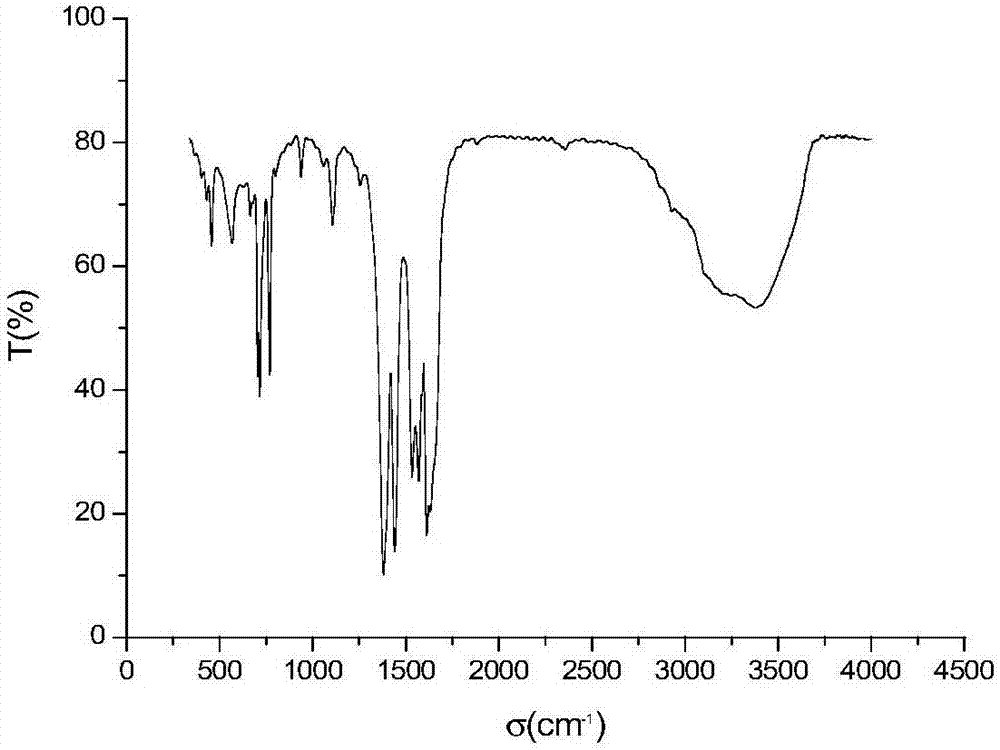
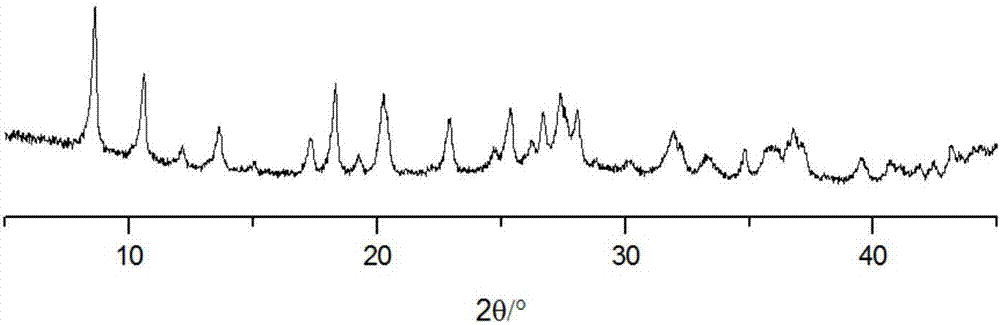
[0029] (3) The turbid solution obtained in step (2) was centrifuged, washed with DMF and methanol respectively, centrifuged (4500 rpm×10 min), and vacuum-dried at 150° C. to obtain the LOFs material.
[0030] (4) Add a certain amount of LOFs material prepared in step (3) into an aqueous solution containing a certain concentration of imazethapyr enantiomer of the chiral pe...
Embodiment 2
[0035] (1) Weigh a certain amount of lanthanide nitrate hexahydrate (Ln(NO 3 ) 3 ·6H 2 O, Ln=Tb and Eu) and organic ligands (trimesic acid BTC and L-aspartic acid L-Asp), then add a certain amount of N,N-dimethylformamide (DMF), ethanol and water, shake well to dissolve and form a mixed solution. Ln(NO 3 ) 3 ·6H 2 The ratio of O to organic ligand is 1mmol:1.5mmol; the ratio of Eu to Tb is 1mmol:9.0mmol; the ratio of BTC to L-Asp is 1mmol:1mmol.
[0036] (2) Place the above mixed solution in a quartz microwave reaction tube, heat the reaction by microwave at 100°C for 5min, and cool to room temperature;
[0037] (3) The turbid solution obtained in step (2) was centrifuged, washed with DMF and methanol respectively, centrifuged (4500 rpm×10 min), and vacuum-dried at 150° C. to obtain LOFs material.
[0038] (4) Adding a certain amount of LOFs material prepared in step (3) into an aqueous solution containing a certain concentration of imazethapyr enantiomer of the chiral p...
Embodiment 3
[0043] (1) Weigh a certain amount of lanthanide nitrate hexahydrate (Ln(NO 3 ) 3 ·6H 2 O, Ln=Tb and Eu) and organic ligands (trimesic acid BTC and L-aspartic acid L-Asp), then add a certain amount of N,N-dimethylformamide (DMF), ethanol and water, shake well to dissolve and form a mixed solution. Ln(NO 3 ) 3 ·6H 2 The ratio of O to organic ligand is 1mmol:2.0mmol; the ratio of Eu to Tb is 1mmol:9.5mmol; the ratio of BTC to L-Asp is 1mmol:1mmol.
[0044] (2) Place the above mixed solution in a quartz microwave reaction tube, microwave heating reaction at 150°C for 5min, and cool to room temperature;
[0045] (3) The turbid solution obtained in step (2) was centrifuged, washed with DMF and methanol respectively, centrifuged (4500 rpm×10 min), and vacuum-dried at 150° C. to obtain LOFs material.
[0046] (4) Adding a certain amount of LOFs material prepared in step (3) into an aqueous solution containing a certain concentration of imazethapyr enantiomer of the chiral pesti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com