Epoxide hydrolase sourced from Phaseolus vulgaris and application thereof
A technology of epoxide and hydrolase, which is applied in the biological field to achieve the effect of strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
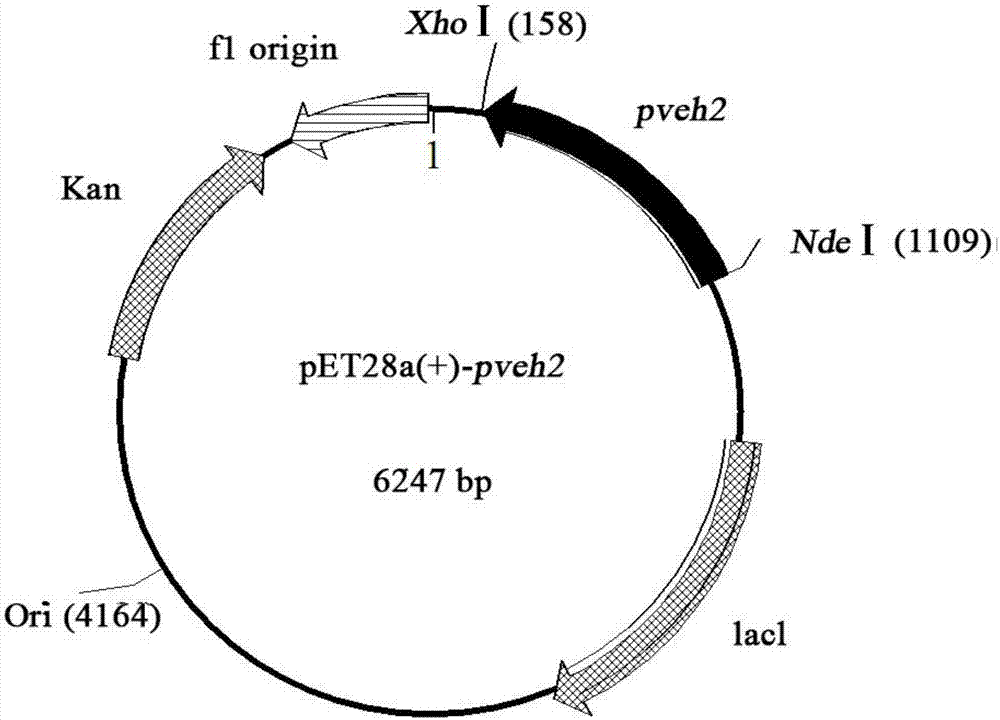
[0028] The cloning of embodiment 1 epoxide hydrolase gene and the construction of expression plasmid
[0029] The total RNA of kidney bean was extracted (using TRIZOL kit, Invitrogen Company), and the operation was performed according to the instructions of the kit. The reverse transcription process refers to the RT-PCR kit PCR PrimeScriptTM RT reagent Kit with gDNAEraser (Perfect Real Time) manual of Takara Company, and then the cDNA obtained by reverse transcription is used as a template, and the upstream and downstream primer sequences are respectively SEQ ID NO: 3 and SEQ ID NO.4 (Nde I and Xho I restriction endonuclease sites were introduced into the primers, and the primers were all synthesized by Shanghai Shenggong Co., Ltd.), the pveh2 full-length gene was amplified by conventional PCR method, and its nucleotide The sequence is shown as SEQ ID NO.1, and the PCR reaction conditions are: pre-denaturation at 94°C for 2 minutes, denaturation at 94°C for 30 seconds, anneali...
Embodiment 2
[0030] The preparation of embodiment 2 epoxide hydrolase PvEH2
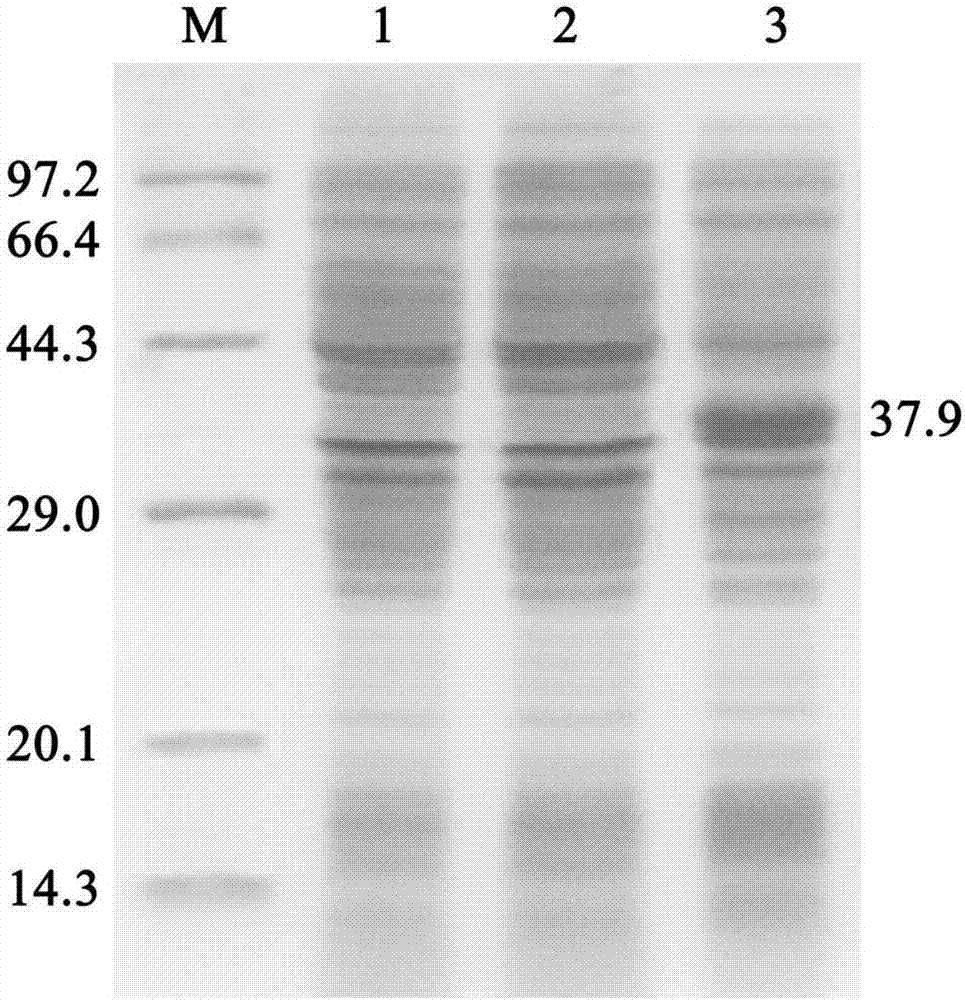
[0031] The genetically engineered bacteria E.coli BL21(DE3) / pET28a(+)-pveh2 in Example 1 was inoculated in LB liquid medium (containing 0.1mg / ml kanamycin sulfate), and cultivated at 37°C for 10~16h ( Optimizing, be 12h), then transfer in the LB culture medium that contains same composition with the inoculum size of 1% (v / v) to continue expanding culture, when thalline concentration OD 600 When the value reaches 0.8-1.0 (optimized, 0.8), add IPTG to its final concentration of 0.2mmol / l, induce culture at 25°C for 4-9h (optimized, 7h), and then centrifuge at 8000r / min for 5min at 4°C , collect the bacterial cell precipitate, and according to the above steps, 0.5-0.8 g of wet bacterial cell can be obtained by fermentation per 100 ml of culture medium. The results of SDS-PAGE analysis of bacterial cells are as follows: figure 2 As shown, the apparent molecular weight of the target protein is about 37.9kDa. .
Embodiment 3
[0032] Example 3 Application of Epoxide Hydrolase PvEH2 in the Preparation of Chiral Styrene Oxide
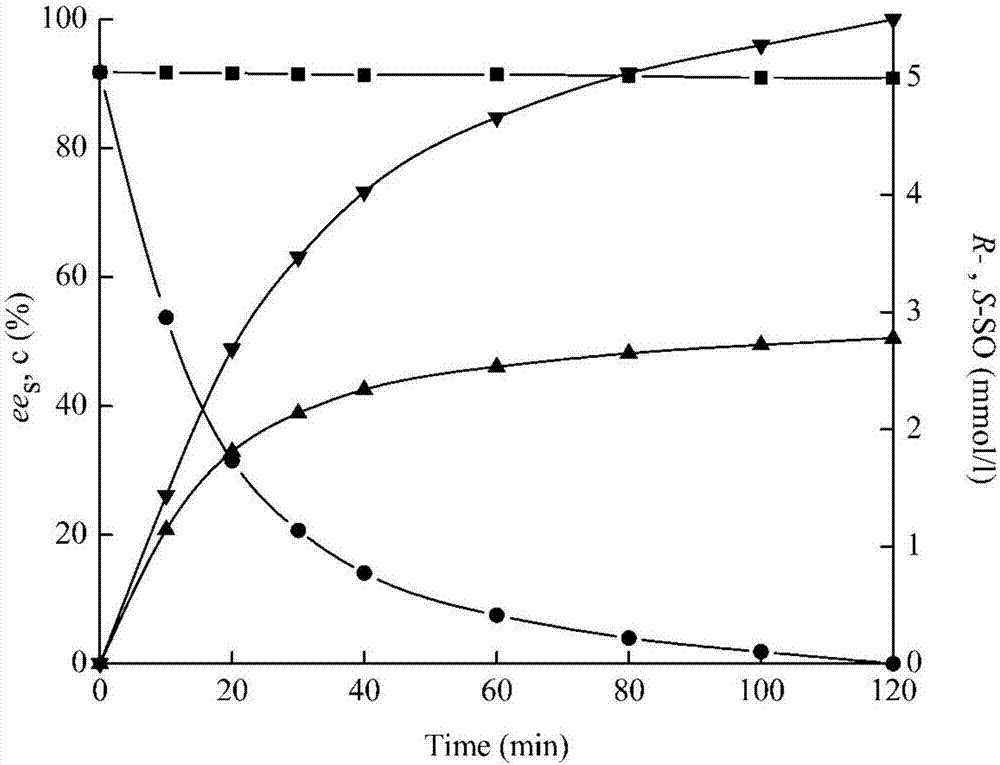
[0033] The wet thalli obtained through centrifugation in embodiment 2 is according to every g corresponding to 10ml NaH 2 PO 4 -Na 2 HPO 4 (100mM, pH7.2) buffer ratio re-suspended, bacterial cell suspension directly as a biological enzyme catalyst, racemic styrene oxide as a substrate for conversion to prepare R-type styrene oxide. The specific operation is: add 2.5ml bacterial cell suspension (bacterial concentration 50mg / ml, activity is 0.04U / ml), 2.25ml buffer solution, 0.25ml racemic epoxy with a concentration of 200mmol / l in a 10ml Ep plastic tube Phenylethane (final concentration in the catalytic system is 10 mmol / l) was converted at 25° C. for 120 min while shaking, and finally the same volume of ethyl acetate was added to extract and terminate the reaction.
[0034] The reaction process of PvEH2 under the above conditions for styrene oxide is as follows image 3 sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com