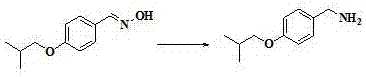Preparation method of Pimavanserin
A technology of pimavanserin and reaction temperature, applied in the direction of organic chemistry, can solve the problems of unfavorable environmental protection, low safety, expensive palladium carbon, etc., and achieve environmental protection, improve safety, and good safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
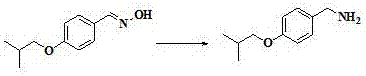
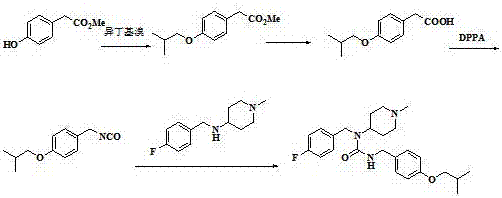
[0026] The preparation method of this pimavanserin is to use 4-isobutoxybenzaldehyde as the starting material, react with hydroxylamine hydrochloride to generate 4-isobutoxybenzaldehyde oxime, and then use zinc powder to dissolve 4-isobutoxybenzaldehyde The reduction of benzaldoxime produces 4-isobutoxybenzylamine, followed by the reaction of 4-isobutoxybenzylamine with diphenyl carbonate to give N-(4-isobutoxybenzyl)phenylcarbamate, and finally with N -(4-fluorobenzyl)-1-methylpiperidin-4-amine reacts to obtain pimavanserin; its synthetic route is as follows:
[0027]
[0028] The process of each step is as follows:
[0029] (1) In the reactor, add hydroxylamine hydrochloride and lye to the water, the lye is sodium hydroxide solution or potassium carbonate solution, preferably sodium hydroxide solution; slowly add 4-isobutyric acid dissolved in the solvent dropwise under stirring phenylbenzaldehyde, the solvent is ethanol or THF (tetrahydrofuran), preferably ethanol; the ...
Embodiment 1
[0033] Embodiment 1: The specific process of the preparation method of this pimavanserin is as follows.
[0034] (1) Preparation of 4-isobutoxybenzaldehyde oxime:
[0035]
[0036] Hydroxylamine hydrochloride (15.6g, 0.224mol) and sodium hydroxide (8.98g, 0.224mol) were added to 100g of water at room temperature, stirred for 15 minutes, and 4-isobutoxybenzaldehyde dissolved in 150mL of ethanol was slowly added dropwise (20g, 0.112mol), reacted at room temperature for 40min, TLC detected the reaction, after the reaction, distilled off ethanol under reduced pressure, extracted with water (250mL) and dichloromethane (250mL), separated the organic layer, the water layer was dichloromethane Wash with methane (100mL*2), combine the organic phases, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, filter, and distill off dichloromethane under reduced pressure to obtain crude 4-isobutoxybenzaldehyde oxime. Recrystallize the crude product, add petroleu...
Embodiment 2
[0050] Embodiment 2: The specific process of the preparation method of this pimavanserin is as follows.
[0051] (1) The preparation of 4-isobutoxybenzaldehyde oxime, except following difference, all the other are the same as embodiment 1:
[0052] The dosage of hydroxylamine hydrochloride is 11.7g, 0.168mol, that is, 4-isobutoxybenzaldehyde: hydroxylamine hydrochloride=1:1.5 (mol); the reaction temperature of 4-isobutoxybenzaldehyde and hydroxylamine hydrochloride is 20-25°C. 19.51g of the product was obtained with a yield of 90%.
[0053] The NMR data of the obtained 4-isobutoxybenzaldoxime are as follows: H1NMR (500MHz, DMSO) δ8.042 (s.1H), 7.482-7.499 (d.2H), 6.923-6.940 (d, 2H), 3.744-3.757 (d.2H), 1.970-2.049(m,1H), 0.969-0.983(d,6H).
[0054] (2) The preparation of 4-isobutoxybenzylamine is the same as in Example 1 except for the following differences:
[0055] The amount of zinc powder is 81.24g, 1.236mol, that is, 4-isobutoxybenzylaldoxime:zinc powder=1:12 (mol); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com