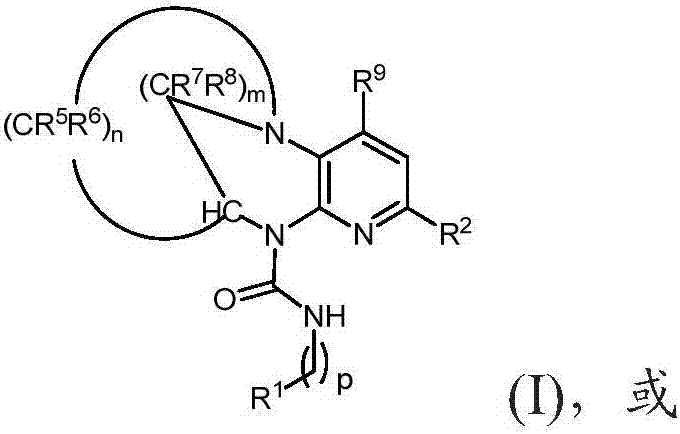
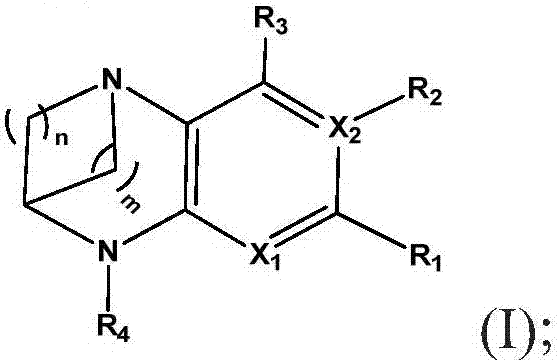
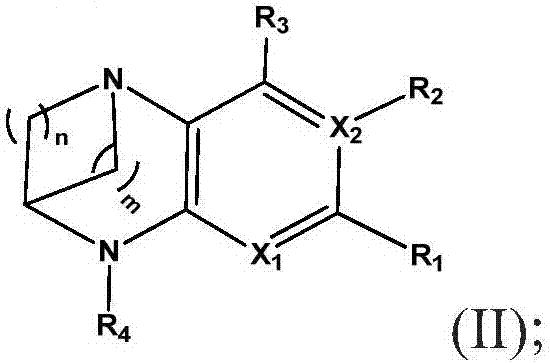
Substituted bridged urea analogs as sirtuin modulators
A compound, heterocyclic group technology, applied in the field of substituted bridged urea analogs as sirtuin regulators, can solve problems such as reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1225] Synthesis of (8S)-N9-(4-((R)-2,3-dihydroxypropoxy)pyridin-2-yl)-4-methyl-N2-((R)-1,1,1- Trifluoroprop-2-yl)-7,8-dihydro-5,8-methylenepyrimidino[4,5-b][1,4]diazepine -2,9(6H)-Diformamide
[1226]
[1227] To (8S)-N9-(4-(((S)-2,2-dimethyl-1,3-dioxolane-4-yl)methoxy)pyridine-2- Yl)-4-methyl-N2-((R)-1,1,1-trifluoroprop-2-yl)-7,8-dihydro-5,8-methylenepyrimidino[4,5 -b][1,4]diazepine To a stirred solution of -2,9(6H)-dimethylamide (350 mg, 0.619 mmol) in methanol (5 mL) was added aqueous HCl solution (1.5 mL, 18.00 mmol) and the reaction mixture was stirred at 28°C for 2 h. (TLC eluent: 5% MeOH in DCM, R f : 0.3), then the solvent is evaporated and the resulting residue is NaHCO 3 The solution was neutralized, the resulting solid was filtered and triturated with pentane (20 mL), and dried under reduced pressure to obtain the desired product (8S)-N9-(4-((R)-2,3-dihydroxypropoxy) Pyridin-2-yl)-4-methyl-N2-((R)-1,1,1-trifluoroprop-2-yl)-7,8-dihydro-5,8-methylenepyrimidino [4,5...
Embodiment 2
[1230] Synthesis of (8S)-N9-(1-methyl-2-oxo-1,2-dihydropyridin-3-yl)-N2-(2,2,2-trifluoroethyl)-7,8- Dihydro-5,8-methylenepyrimido[4,5-b][1,4]diazepine -2,9(6H)-Diformamide
[1231]
[1232] To (8S)-9-((1-methyl-2-oxo-1,2-dihydropyridin-3-yl)carbamoyl)-6,7,8,9-tetrahydro-5 , 8-methylenepyrimido[4,5-b][1,4]diazepine Add DIPEA (0.490mL, 2.81mmol), HATU (46.8mg, 0.123mmol) and 2,2,2-trifluoroethane to a stirred solution of -2-formic acid (200mg, 0.561mmol) in DMF (2mL) at once Amine (55.6 mg, 0.561 mmol). The reaction mixture was stirred at room temperature for 16 h. (TLC eluent: 5% MeOH in DCM: R f -0.5; UV activity). The reaction mixture was diluted with cold water and extracted with ethyl acetate (2x 50ml). The combined organic layer was washed with brine solution (20 mL) and washed with anhydrous Na 2 SO 4 Dry, filter and concentrate under reduced pressure to obtain crude product. The crude product was purified by combi flash chromatography (using silica gel column 12g, el...
Embodiment 3
[1235] Synthesis of (8S)-N-(pyridin-2-yl)-2-(3-(trifluoromethyl)phenyl)-7,8-dihydro-5,8-methylenepyrimido[4,5 -b][1,4]diazepine -9(6H)-formamide
[1236]
[1237] To (8S)-2-(3-(trifluoromethyl)phenyl)-6,7,8,9-tetrahydro-5,8-methylenepyrimido[4,5-b] at 0℃ [1,4] Diaza (550mg, 1.796mmol), 3-(pyridin-2-yl)-2H-pyrido[1,2-a][1,3,5]triazine-2,4(3H)-dione (863mg, 3.59 mmol) Sodium hydride (359 mg, 8.98 mmol) was added to a solution in THF (15 mL). The reaction mixture was heated to 60°C for 16 h. It was returned to room temperature and the reaction mixture was poured into cold water (100 mL), and extracted with ethyl acetate (2x200 mL). Use anhydrous Na for the combined organic layer 2 SO 4 Dry and concentrate under reduced pressure to obtain a crude product. The crude product mixture was purified by flash column chromatography (silica gel: 100-200 mesh, using a gradient mixture of 70 to 100% ethyl acetate in petroleum ether) to obtain 400 mg of a semi-pure compound with 91% purity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com