A traditional Chinese medicine composition for preventing and treating rheumatoid arthritis and its preparation method
A composition and arthritic technology are applied in the field of traditional Chinese medicine compositions for preventing and treating rheumatoid arthritis and the field of preparation thereof, which can solve the problems of long treatment time and slow effect, achieve less harsh process conditions, reduce slow absorption and irregularity, The effect of facilitating the promotion of the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Weigh 6 grams of Eucommia ulmoides, 6 grams of Morus vulgaris, 7 grams of Weilingxian, 6 grams of Salvia miltiorrhiza, 5 grams of Earth Dragon, 8 grams of Habitat, 6 grams of Papaya, 6 grams of Snake Snake, 5 grams of Panax notoginseng, 5 grams of Solanum, 5 grams of safflower, 6 grams of white peony, 6 grams of spatholobi, 7 grams of angelica, 3 grams of centipede.
[0052] Step 2. Preparation of alcohol extract
[0053] Add the weighed peony root, Eucommia ulmoides, Weilingxian, Morus sylvestris, Salvia miltiorrhiza, Panax notoginseng, Safflower, and Safflower to 75% ethanol, then reflux and extract 3 times at 90°C to obtain an alcohol extract, and reflux 75% ethanol for 3 times The amount of addition is: the first time is 8 times the total mass of white peony, Eucommia ulmoides, Weilingxian, Morus sylvestris, Salvia miltiorrhiza, Panax notoginseng, Radix sylvestris, and safflower, the second time is 8 times the total mass of the above , The third time is 6 times the sum ...
Embodiment 2
[0061] 1. Investigation of the therapeutic activity of the traditional Chinese medicine composition of the present invention.
[0062] 1) Animal grouping and adjuvant arthritis rat model construction.
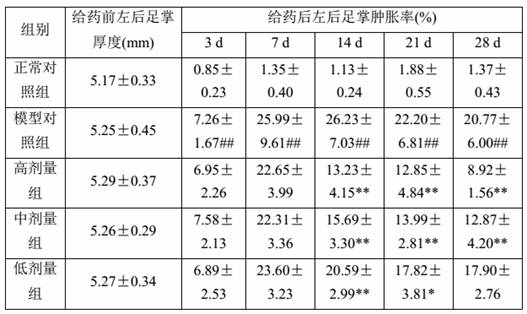
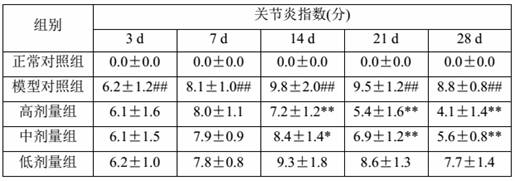
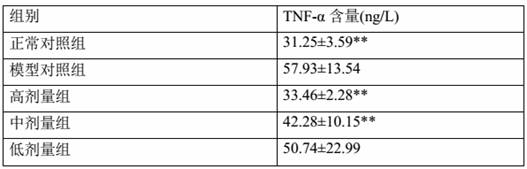
[0063] After 2 weeks of adaptive feeding, 10 SD rats were randomly selected as the normal control group, and the rest were used for model building. After fasting for 12 hours, except the normal control group, the rest of the rats were placed in cold water at 4°C-8°C with a water surface height of 2 cm. After 30 minutes, they were taken out. The body of the rat was dried by an electric fan, and the rat’s right hind foot pad Intradermal injection of Freund’s complete adjuvant 0.1 ml / mouse, rats in the normal group were injected with an equal volume of normal saline for 2 consecutive days. On the 9th day after the inflammation, the rats developed secondary arthritis symptoms (non-inflammatory and joint swelling of both forelimbs), listlessness, and loose hair in the left hind foot, in...
Embodiment 3
[0081] Comparison of the therapeutic effects of different dosage forms on cotton ball granuloma in adjuvant arthritis rats.
[0082] The inventor conducted a comparative study on the therapeutic effect of the gel and ointment of the Chinese medicine composition of the present invention on the treatment of adjuvant arthritis rats with cotton ball granuloma.
[0083] The components in Example 1 were pulverized, sieved with 120 mesh, and the fine powder was mixed and added with yellow wine, and stirred into a paste to obtain a paste mixture, wherein each 100 g paste mixture contained 30 g of traditional Chinese medicine composition. The obtained mixture is uniformly smeared on the plaster to obtain the plaster of the present invention.
[0084] 40 rats were randomly divided into normal control group, model control group, Chinese medicine gel group and traditional plaster group. The method of building adjuvant arthritis rat model and the method of administration are the same as above. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com