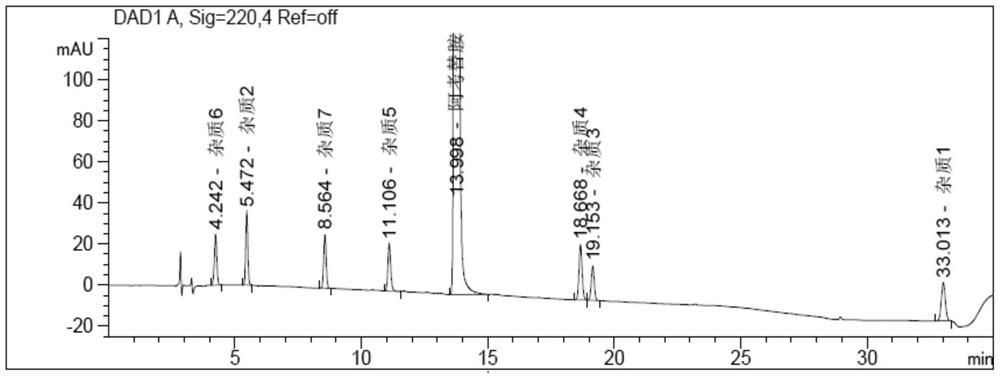
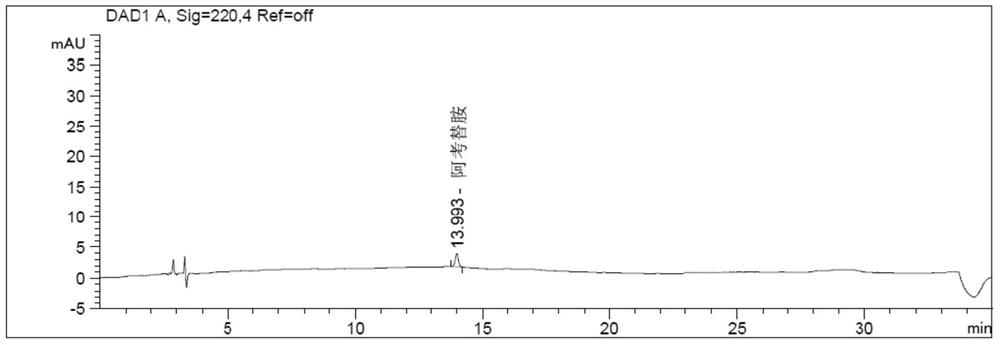
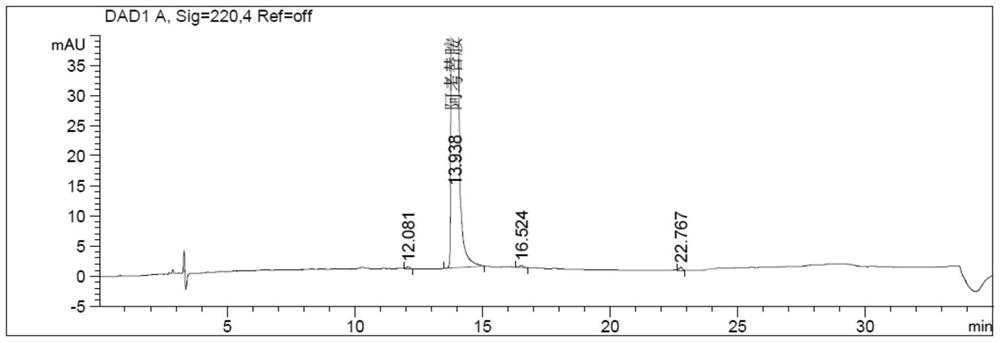
Method for determining related substances in acotiamide hydrochloride bulk drug and its preparations by HPLC
A technology for acotiamide hydrochloride and related substances, applied in the field of drug analysis, can solve problems to be improved, etc., and achieve the effects of good separation, strong operability and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Instrument: Agilent 1260 High Performance Liquid Chromatograph, 1260Infinity Diode Array Detector
[0057] Chromatographic column: Hypersil BDS C8 (250*4.6mm, 5μm)
[0058] Mobile phase A: 0.02mol / L potassium dihydrogen phosphate buffer solution (containing 0.2 volume% triethylamine, adjust pH to 6.0 with concentrated phosphoric acid)
[0059] Mobile Phase B: Acetonitrile
[0060] See the table below for gradient elution:
[0061] time / min Phosphate buffer / % The first organic solvent / % 0 85 15 20 65 35 25 50 50 30 50 50 30.01 85 15 35 85 15
[0062] Flow rate: 1.0mL / min
[0063] Detection wavelength: 220nm
[0064] Column temperature: 30°C
[0065] Injection volume: 10μL
[0066] Solvent: acetonitrile-water (30:70)
[0067] Run time: 35 minutes
[0068] 1. Determination of chromatographic conditions
[0069] Acotiamide hydrochloride and its impurity 1 to impurity 7 were scanned by ultraviolet spectrum between...
Embodiment 2
[0086] Instrument: Agilent 1260 High Performance Liquid Chromatograph, 1260Infinity Diode Array Detector
[0087] Chromatographic column: Hypersil BDS C8 (250*4.6mm, 5μm)
[0088] Mobile phase A: 0.02mol / L potassium dihydrogen phosphate buffer solution (containing 0.2 volume% triethylamine, adjust pH to 6.0 with concentrated phosphoric acid)
[0089] Mobile Phase B: Acetonitrile
[0090] See the table below for gradient elution:
[0091] time / min Phosphate buffer / % The first organic solvent / % 0 85 15 20 65 35 25 50 50 30 50 50 30.01 85 15 35 85 15
[0092] Flow rate: 1.0mL / min
[0093] Detection wavelength: 220nm
[0094] Column temperature: 30°C
[0095] Injection volume: 10μL
[0096] Solvent: acetonitrile-water (30:70)
[0097] Run time: 35 minutes
[0098] Experimental steps:
[0099] The test solution: Accurately weigh about 25 mg of acotiamide hydrochloride crude drug, put it in a 50 mL measuring bottle, add a...
Embodiment 3
[0104] Instrument: Agilent 1260 High Performance Liquid Chromatograph, 1260Infinity Diode Array Detector
[0105] Chromatographic column: Hypersil BDS C8 (250*4.6mm, 5μm)
[0106] Mobile phase A: 0.02mol / L potassium dihydrogen phosphate buffer solution (containing 0.2 volume% triethylamine, adjust pH to 6.0 with concentrated phosphoric acid)
[0107] Mobile Phase B: Acetonitrile
[0108] See the table below for gradient elution:
[0109] time / min Phosphate buffer / % The first organic solvent / % 0 85 15 20 65 35 25 50 50 30 50 50 30.01 85 15 35 85 15
[0110] Flow rate: 1.0mL / min
[0111] Detection wavelength: 220nm
[0112] Column temperature: 30°C
[0113] Injection volume: 10μL
[0114] Solvent: acetonitrile-water (30:70)
[0115] Run time: 35 minutes
[0116] Experimental steps:
[0117] Need testing solution: Accurately weigh an appropriate amount of acotiamide hydrochloride tablet fine powder (equivalent to about 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com