Epinastine hydrochloride transdermal patch and preparation method thereof
A technology of epinastine hydrochloride transdermal and epinastine hydrochloride, which is applied in the field of drug preparation, can solve the problems of epinastine hydrochloride bitter taste, poor patient compliance, and restriction of drug use, and achieve good uniformity, Good for absorption and good skin tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
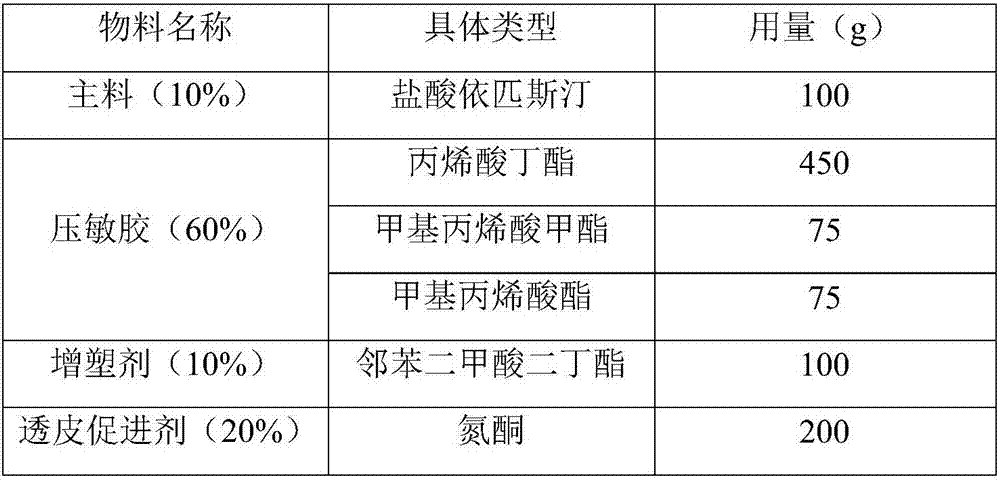
[0039] The prescription of epinastine hydrochloride patch drug storage layer is as follows table 1:
[0040] The consumption of each component of table 1 (total amount 1000g meter)
[0041]
[0042]Preparation method: Weigh each material according to the prescription amount, add methyl phthalate and azone into ethanol and stir well, then add epinastine hydrochloride, methyl methacrylate, butyl acrylate, methacrylate , Stir in a water bath at 60°C for 4 hours until completely mixed, degas after stirring, dry in an oven, apply to an anti-adhesive material to dry, cover with a liner, cut and measure, test, pack, and seal for storage.
Embodiment 2
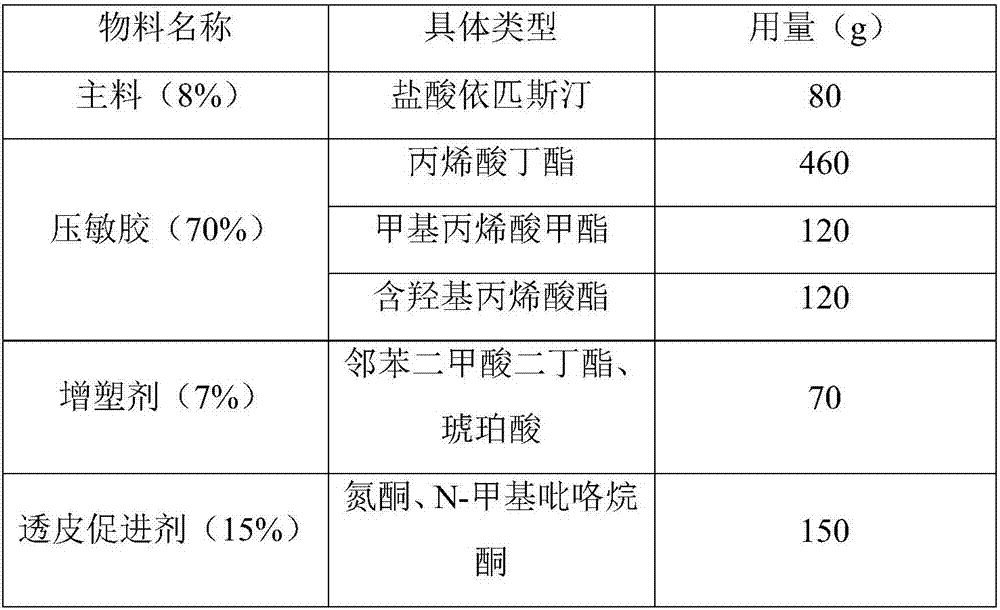
[0044] The prescription of epinastine hydrochloride patch drug storage layer is as follows table 2:
[0045] The dosage of each component in table 2 (total amount 1000g meter)
[0046]
[0047] Preparation method: Weigh each material according to the prescription amount, add methyl phthalate, succinic acid, azone, and N-methylpyrrolidone into ethanol and stir well, then add epinastine hydrochloride, methyl methacrylate , butyl acrylate, hydroxyl-containing acrylate, stirred in a water bath at 70°C for 4 hours until completely mixed at a speed of 300rpm, degassed after stirring evenly, dried in an oven at a drying temperature of 150°C, coated on an anti-adhesive material to dry, covered Lining layer, cutting and sub-measurement, testing, packaging, sealing and storage.
Embodiment 3
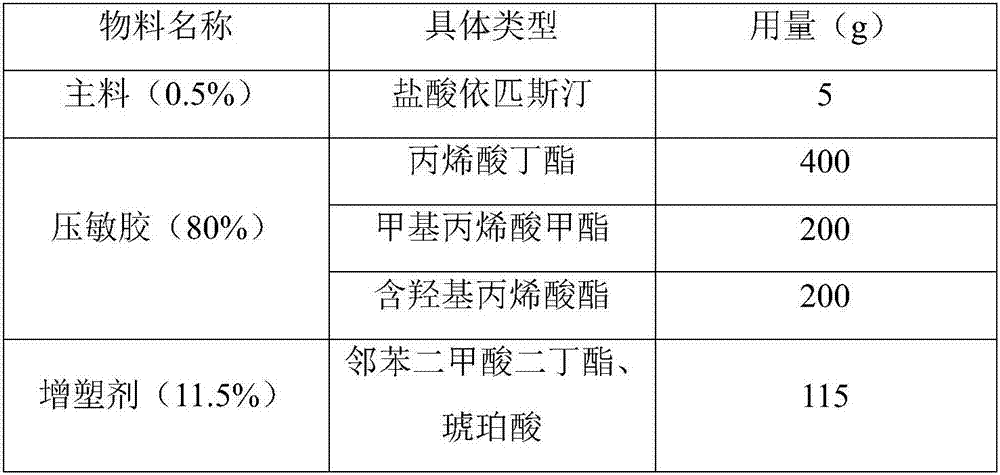
[0049] The prescription of epinastine hydrochloride patch drug storage layer is as follows table 3:
[0050] The consumption of each component of table 3 (total amount 1000g meter)
[0051]
[0052]
[0053] Preparation method: Weigh each material according to the prescription amount, add methyl phthalate, succinic acid, azone, and N-methylpyrrolidone into ethanol and stir well, then add epinastine hydrochloride, methyl methacrylate , butyl acrylate, methacrylate, stirred in a water bath at 70°C for 4 hours until completely mixed at a rate of 700rpm, degassed after stirring evenly, dried in an oven at a drying temperature of 100°C, coated on an anti-adhesive material to dry, covered Lining layer, cutting and sub-measurement, testing, packaging, sealing and storage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com