1,4-hexaethyl butanediamine hydroxide, preparation method and application
A technology of butanediamine hydroxide and hexaethyldibromobutylamine, applied in 1 field, can solve problems such as high price, difficult preparation, unfavorable industrial production, etc., and achieve the effects of reducing synthesis price, simple synthesis process and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
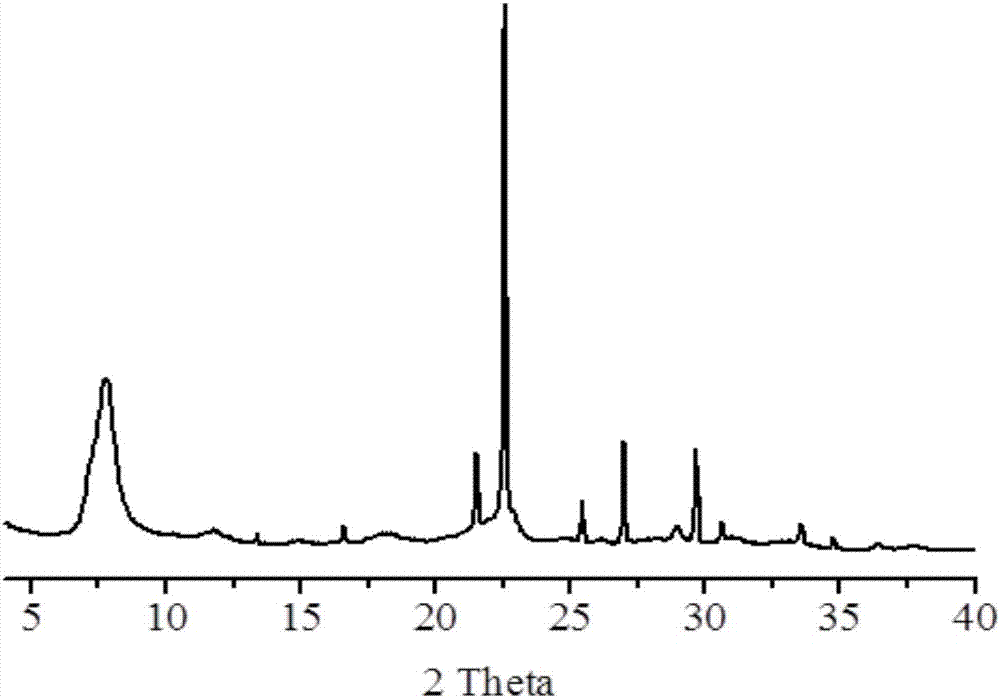
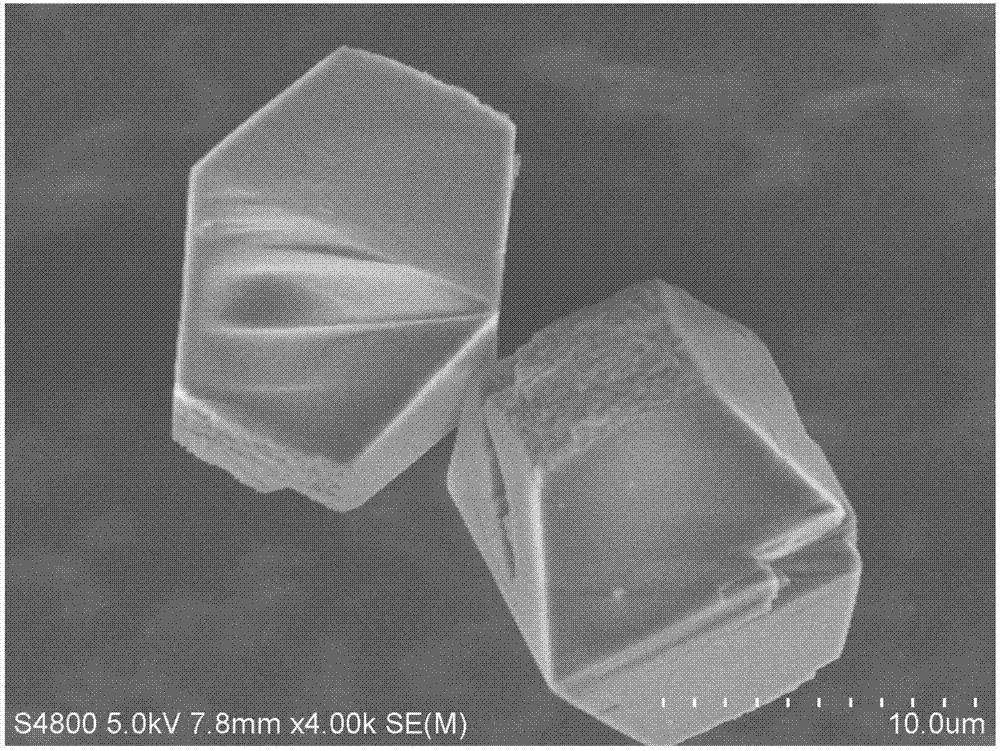
Image
Examples
Embodiment 1
[0034] 1,4-hexaethylbutanediamine hydroxide (1,4-(C 2 h 5 ) 3 NC 4 h 8 N(C 2 h 5 ) 3 (OH) 2 ), its preparation method comprises:
[0035] 1) Mix triethylamine and 1,4-dibromobutane in acetone solution, heat under reflux for 24 hours to obtain a yellowish solid, wash the solid with acetone until white, and the obtained white solid is 1,4-hexaethyldi Bromobutylamine;
[0036]2) Dissolve the white solid powder of 1,4-hexaethyldibromobutylamine in water to obtain a clear solution, and obtain 1,4- Hexaethylbutylenediamine Hydroxide.
[0037] In step 1), the molar ratio of triethylamine to 1,4-dibromobutane is 2.4:1, and the molar ratio of triethylamine to acetone is 0.5:1;
[0038] In step 2), the molar ratio of water to 1,4-hexaethyldibromobutylamine is 60:1;
[0039] The production cycle of the above method for preparing 1,4-hexaethyl butanediamine hydroxide is no more than 48 hours, and the yield of 1,4-hexaethyl dibromobutylamine is 86%. 1,4-hexaethyl hydroxide The...
Embodiment 2
[0041] 1,4-hexaethylbutanediamine hydroxide (1,4-(C 2 h 5 ) 3 NC 4 h 8 N(C 2 h 5 ) 3 (OH) 2 ), its preparation method comprises:
[0042] 1) Mix triethylamine and 1,4-dibromobutane in acetone solution, heat under reflux for 24 hours to obtain a yellowish solid, wash the solid with acetone until white, and the obtained white solid is 1,4-hexaethyldi Bromobutylamine;
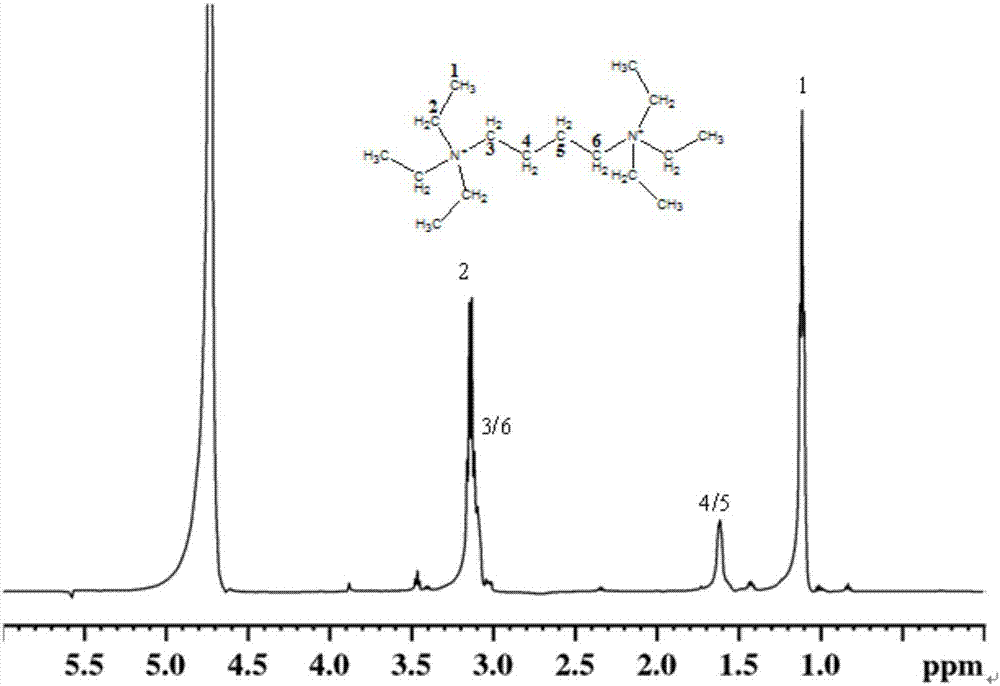
[0043] 2) Dissolve the white solid powder of 1,4-hexaethyldibromobutylamine in water to obtain a clear solution, and obtain 1,4- Hexaethylbutylene diamine hydroxide ( figure 1 ), for Examples 4-7.
[0044] In step 1), the molar ratio of triethylamine to 1,4-dibromobutane is 2.2:1, and the molar ratio of triethylamine to acetone is 0.5:1;
[0045] In step 2), the molar ratio of water to 1,4-hexaethyldibromobutylamine is 65:1;
[0046] The above-mentioned method prepares 1, the production period of 4-hexaethyl butanediamine hydroxide is no more than 48 hours, and the productive rate of 1, 4-hexaethyl di...
Embodiment 3
[0048] 1,4-hexaethylbutanediamine hydroxide (1,4-(C 2 h 5 ) 3 NC 4 h 8 N(C 2 h 5 ) 3 (OH) 2 ), its preparation method comprises:
[0049] 1) Mix triethylamine and 1,4-dibromobutane in acetone solution, heat under reflux for 24 hours to obtain a yellowish solid, wash the solid with acetone until white, and the obtained white solid is 1,4-hexaethyldi Bromobutylamine;
[0050] 2) Dissolve the white solid powder of 1,4-hexaethyldibromobutylamine in water to obtain a clear solution, and obtain 1,4- Hexaethylbutylenediamine Hydroxide.
[0051] In step 1), the molar ratio of triethylamine to 1,4-dibromobutane is 2.0:1, and the molar ratio of triethylamine to acetone is 0.8:1;
[0052] In step 2), the molar ratio of water to 1,4-hexaethyldibromobutylamine is 100:1;
[0053] The production cycle of the above method for preparing 1,4-hexaethyl butanediamine hydroxide is no more than 48 hours, and the yield of 1,4-hexaethyl dibromobutylamine is 80%. 1,4-hexaethyl hydroxide T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com