PCR detection method for human hsa-miR-124a-3p and application of PCR detection method to DS (Down's syndrome) pregnancy rick prediction
A detection method and risk prediction technology, applied in the field of molecular biology, can solve the problems of long inspection cycle, high condition requirements, complex technical operation, etc., and achieve the effects of wide detection linear range, high detection sensitivity and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1, RT-qPCR detection of hsa-miR-124a-3p
[0032] 1. hsa-miR-124a-3p RT reaction
[0033] (1) Synthesis of hsa-miR-124a-3p standard product: log on to http: / / www.mirbase.org / website, obtain the base sequence of hsa-miR-124a-3p as 5′-UAAGGCACGCGGUGAAUGCC-3′ (such as shown in SEQ ID NO:1). The obtained hsa-miR-124a-3p sequence was sent to Dalian Bao Biological Engineering Co., Ltd. to synthesize hsa-miR-124a-3p standard (12.8nmols).
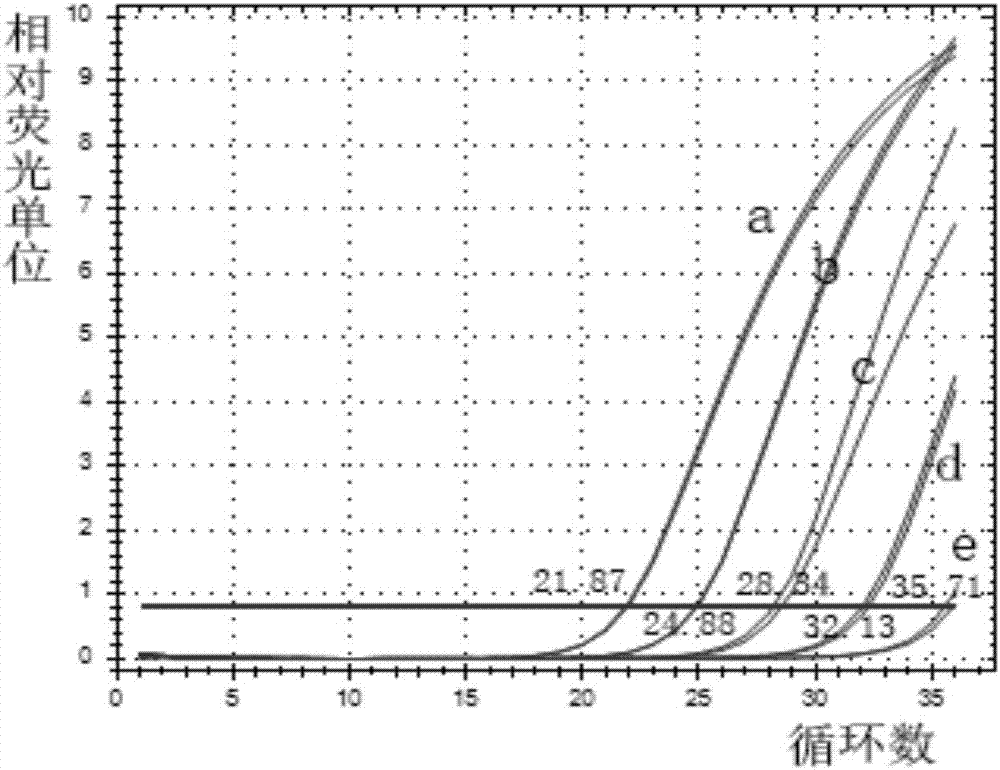
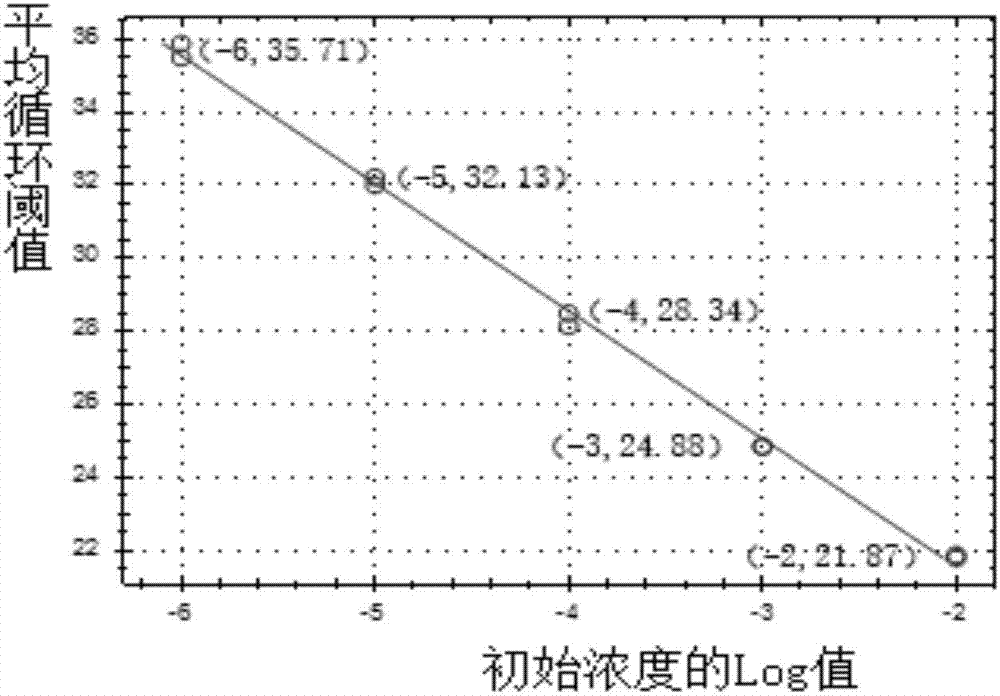
[0034] (2) Preparation of hsa-miR-124a-3p standard products with different concentrations: add 1.28ml RNase-free water to 12.8nmol hsa-miR-124a-3p standard products to prepare 10pmol / μL hsa-miR- 124a-3p standard solution, as a storage solution for the standard. Then continue to use RNase-free water to dilute the 10pmol / μL hsa-miR-124a-3p standard to 10-fold concentration gradient to two concentrations of 1 and 0.1pmol / μL.
[0035] (3) Design of hsa-miR-124a-3p RT primers: Replace all U in the hsa-miR-124a-3p sequence with T to ob...
Embodiment 2
[0054] Example 2. Clinical application of maternal plasma hsa-miR-124a-3p in prenatal screening for Down syndrome
[0055] 1. Extraction of total RNA in plasma
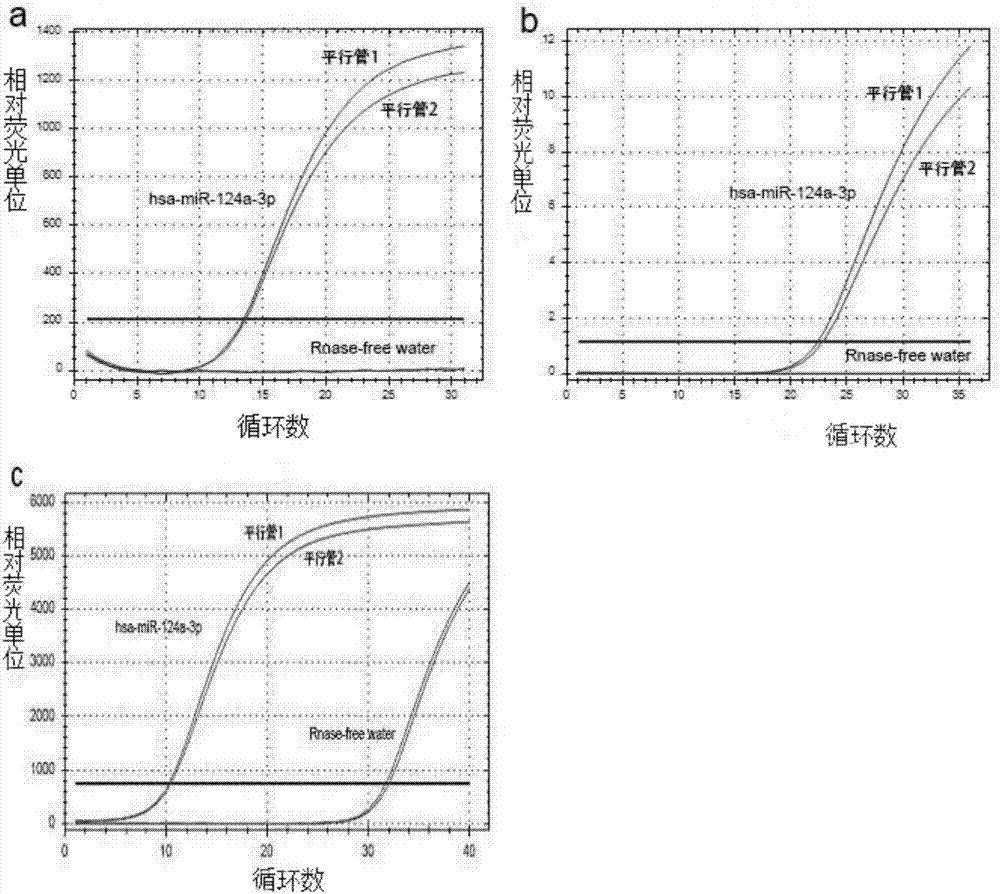
[0056] Collection and grouping of clinical specimens: 36 cases of plasma from pregnant women in the second trimester (13-27 weeks) of healthy fetuses aged 20-35 were collected from the Prenatal Diagnosis Center of the Department of Obstetrics and Gynecology, the First Affiliated Hospital of Third Military Medical University, and they were used as the normal control group; DS was diagnosed in pregnancy 11 cases of pregnant women's plasma in the second trimester of pregnancy belonged to the DS pregnancy group; the blood samples were anticoagulated with EDTA, centrifuged at 3000r / min at 25°C for 3min, and the upper layer of plasma was drawn, and stored at -80°C for later use. Total RNA was extracted from plasma.
[0057] 2. RT-qPCR reaction
[0058] According to the method in Example 1, RT-qPCR was performed on the ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com