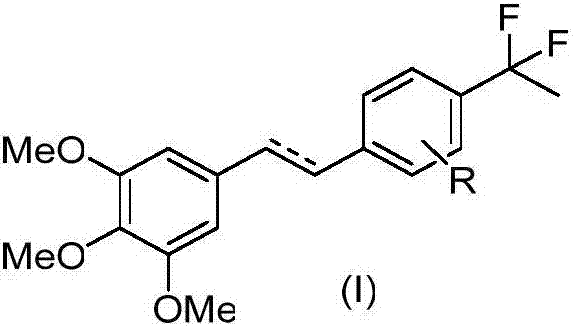
Gem-difluoro ethyl substitutional diphenylethene and diphenylethane derivative as well as preparation method and application thereof
A technology of stilbene and difluoroethyl, applied in the field of drug synthesis to achieve the effect of enhancing in vitro anti-tumor activity and strengthening in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Synthesis of 4-(1,1-difluoro)ethylbenzaldehyde
[0023]
[0024] Step 1: Preparation of methyl 4-(1,1-difluoroethyl)benzoate
[0025] Dissolve methyl 4-acetylbenzoate (5.00 g, 28.06 mmol) in 40 mL of anhydrous CH 2 Cl 2 Add to sealed tube, N 2 Protection, slowly add DAST (diethylaminosulfur trifluoride) (12mL, 3.4eq) dropwise at -40°C, and then heat in an oil bath at 60°C for 24h, after the reaction is completed, it will drop below 0°C, and use saturated NaHCO 3 Aqueous solution quenches the reaction, CH 2 Cl 2 Extract, combine the organic phases, concentrate, and separate by column chromatography with EA:PE=1:150 to obtain 3.30 g of white solid with a yield of 59%. Mp:35.2-35.7℃. 1 H NMR (CDCl 3,500MHz): δ1.92(t,3H,J=18.0Hz),3.93(s,3H),7.57(d,2H,J=8.0Hz),8.09(d,2H,J=8.0Hz). 13 C NMR (CDCl 3 ,125MHz): δ166.3,142.6142.4,142.2,131.4,129.8,124.7,123.8,121.4,119.5,52.27,26.1,25.9,25.6. 19 F NMR (CDCl 3 ,470MHz):δ-88.70.MS(m / z):200(M + ).ESI-HRMS(m / z...
Embodiment 2
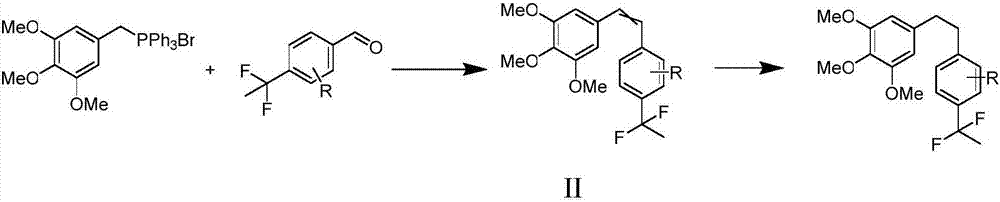
[0030] Preparation of Example 2 (Z / E)-3,4,5-trimethoxy-4'-(1,1-difluoroethyl)stilbene
[0031]
[0032] N 2 Under protection, 3,4,5-trimethoxybenzyl bromidetriphenylphosphine bromide (3.08g, 5.88mmol) was suspended in 50mL of anhydrous THF solution, and n-butyllithium (4.5 mL, 7.06mmol, 1.2eq), then reacted for 1h, then slowly added dropwise a THF solution (5mL) of 4-(1,1-difluoroethyl)benzaldehyde (1.00g, 5.88mmol) to the reaction system, liter Reaction at room temperature overnight for 12h. After the reaction, cool the system to below -5°C, add saturated NaCl aqueous solution, extract with EA, combine the organic phases, anhydrous NaCl 2 SO 4 After drying, filtering and concentrating, column chromatography separated EA:PE=1:25 to obtain 1.20 g of oil as a cis-trans mixture with a yield of 64% (Z:E=4:1). Further separation by column chromatography yielded pure cis-trans isomers.
[0033] (Z)-3,4,5-Trimethoxy-4'-(1,1-difluoroethyl)stilbene
[0034] White oil Z-isomer:...
Embodiment 3
[0037] Example 3 Preparation of 3,4,5-trimethoxy-4'-(1,1-difluoroethyl)diphenylethane
[0038]
[0039] Dissolve (Z / E)-3,4,5-trimethoxy-4'-(1,1-difluoroethyl)stilbene (1.20 g, 3.6 mmol) in 30 mL of ethanol and add Pd / C (0.10g, 0.9mmol), H 2 The excess was stirred overnight at room temperature. After the reaction, the Pd / C was removed by filtration, and the solvent was spin-dried to obtain 1.10 g of a white solid with a yield of 90%. Mp:60.1-60.7℃. 1 H NMR (CDCl 3 ,500MHz):δ1.91(t,3H,J=18.0Hz),2.86(m,2H),2.94(m,2H),3.82(d,9H,J=10.0Hz),6.34(s,2H) ,7.22(d,2H,J=7.5Hz),7.42(d,2H,J=7.5Hz). 13 C NMR (CDCl 3 ,125MHz): δ153.1,143.3,137.1,136.3,136.2,135.9,135.7,128.6,124.7,124.6,124.6,123.8,121.9,120.0,105.5,60.9,56.1,38.1,37.7,29,125.25. 19 F NMR (CDCl 3 ,470MHz):δ-86.84.MS(m / z):336(M + ).ESI-HRMS(m / z): calculated for C 19 h 22 f 2 o 3(M+H) + :337.1615,found:337.1610.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com