Traditional Chinese medicine preparation for treating dizziness, palpitation, insomnia and forgetfulness and preparation method of traditional Chinese medicine preparation
A technology for insomnia, forgetfulness, and traditional Chinese medicine preparations, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve problems such as unsatisfactory and incomplete extraction of active ingredients, and achieve low production costs. , Guarantee the content and curative effect of active ingredients, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] A method for preparing a dispersible tablet from a traditional Chinese medicine preparation for treating dizziness, palpitation, insomnia and amnesia, comprising the following steps:
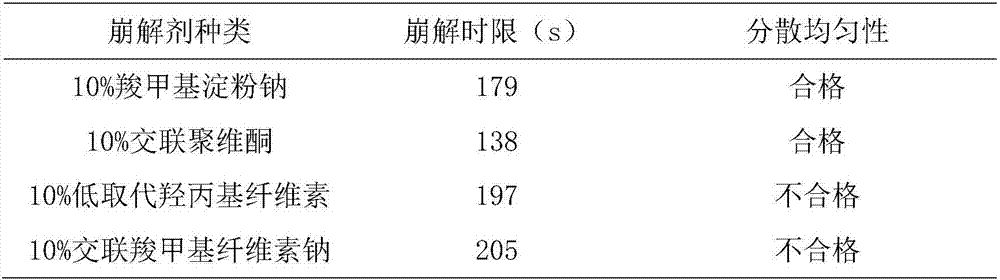
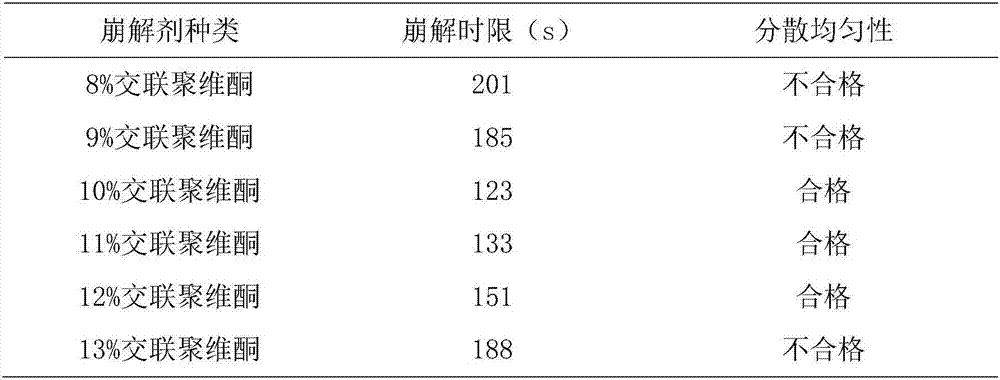
[0082] T1. Take the following components respectively: hydroxypropyl methylcellulose, pregelatinized starch, crospovidone, sodium carboxymethyl starch, sodium lauryl sulfate, micropowder silica gel, pulverize through a 100-mesh sieve, and set aside ;
[0083] T2. Weigh the components according to the following parts by weight: 800g of Agrimony, 400g of Eclipta, 400g of Caulis Spatholobus, 400g of Rehmannia glutinosa, 400g of Rehmannia glutinosa, 400g of Albizia Julibrissin, 450g of Shouwu vine, 300g of sodium alginate, hydroxypropyl methyl Base cellulose 100g, calcium sulfate 200g, crospovidone 200g, dextran 200g, sodium lauryl sulfate 50g, micronized silica gel 10g;
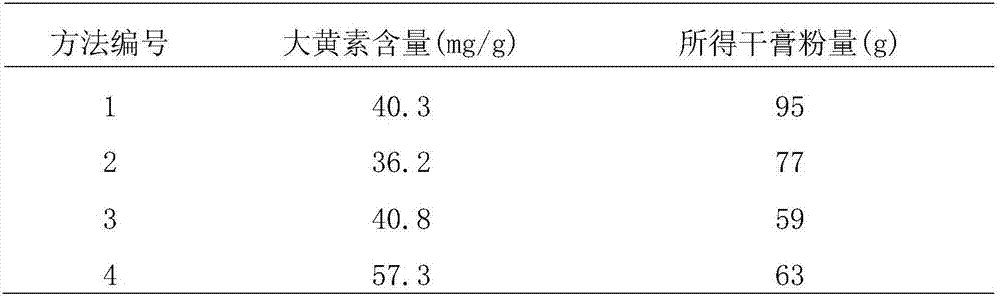
[0084]T3. Preparation of medicinal material extract dry paste powder: get Shouwu vine, add 3 times the amount of 70% eth...
Embodiment 2
[0090] A method for preparing a dispersible tablet from a traditional Chinese medicine preparation for treating dizziness, palpitation, insomnia and amnesia, comprising the following steps:
[0091] T1. Take the following components respectively: hydroxypropyl methylcellulose, pregelatinized starch, crospovidone, sodium carboxymethyl starch, sodium lauryl sulfate, micropowder silica gel, pulverize through a 100-mesh sieve, and set aside ;
[0092] T2. Weigh the components according to the following parts by weight: 1200g Agrimony, 800g Eclipta, 800g Caulis Spatholobus, 800g Rehmannia glutinosa, 800g Rehmannia glutinosa, 800g Albizia Julibrissin, 750g Shouwuteng, 500g sodium alginate, hydroxypropyl methyl Base cellulose 200g, calcium sulfate 400g, crospovidone 300g, dextran 400g, sodium lauryl sulfate 15g, micropowder silica gel 3g;
[0093] T3. Preparation of medicinal material extract dry cream powder: get Shouwu vine, add 5 times the amount of 70% ethanol solution to reflux...
Embodiment 3
[0099] A method for preparing a dispersible tablet from a traditional Chinese medicine preparation for treating dizziness, palpitation, insomnia and amnesia, comprising the following steps:
[0100] T1. Take the following components respectively: hydroxypropyl methylcellulose, pregelatinized starch, crospovidone, sodium carboxymethyl starch, sodium lauryl sulfate, micropowder silica gel, pulverize through a 100-mesh sieve, and set aside ;
[0101] T2. Weigh the components according to the following parts by weight: 1000g of Agrimony, 600g of Eclipta, 600g of Caulis Spatholobus, 600g of Rehmannia glutinosa, 600g of Rehmannia glutinosa, 600g of Albizia Julibrissin, 600g of Shouwu vine, 400g of sodium alginate, hydroxypropyl methyl Base cellulose 150g, calcium sulfate 300g, crospovidone 250g, dextran 300g, sodium lauryl sulfate 10g, micropowder silica gel 2g;
[0102] T3. Preparation of medicinal material extract dry cream powder: get Shouwu vine, add 4 times the amount of 70% e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com