A nitric acid selective leaching method for low-nickel high-iron alloy powder
A ferroalloy and selective technology, applied in the direction of improving process efficiency, can solve the problems of high iron removal cost, cobalt loss, and difficulty in iron removal, and achieve the effects of saving nitric acid consumption, ensuring leaching rate, and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The composition of the low-nickel high-iron alloy powder is shown in Table 1.
[0015] Table 1: Composition of low-nickel high-iron alloy powder (%)
[0016]
[0017] The nitric acid selective leaching method of the low-nickel high-iron alloy powder comprises the following steps:
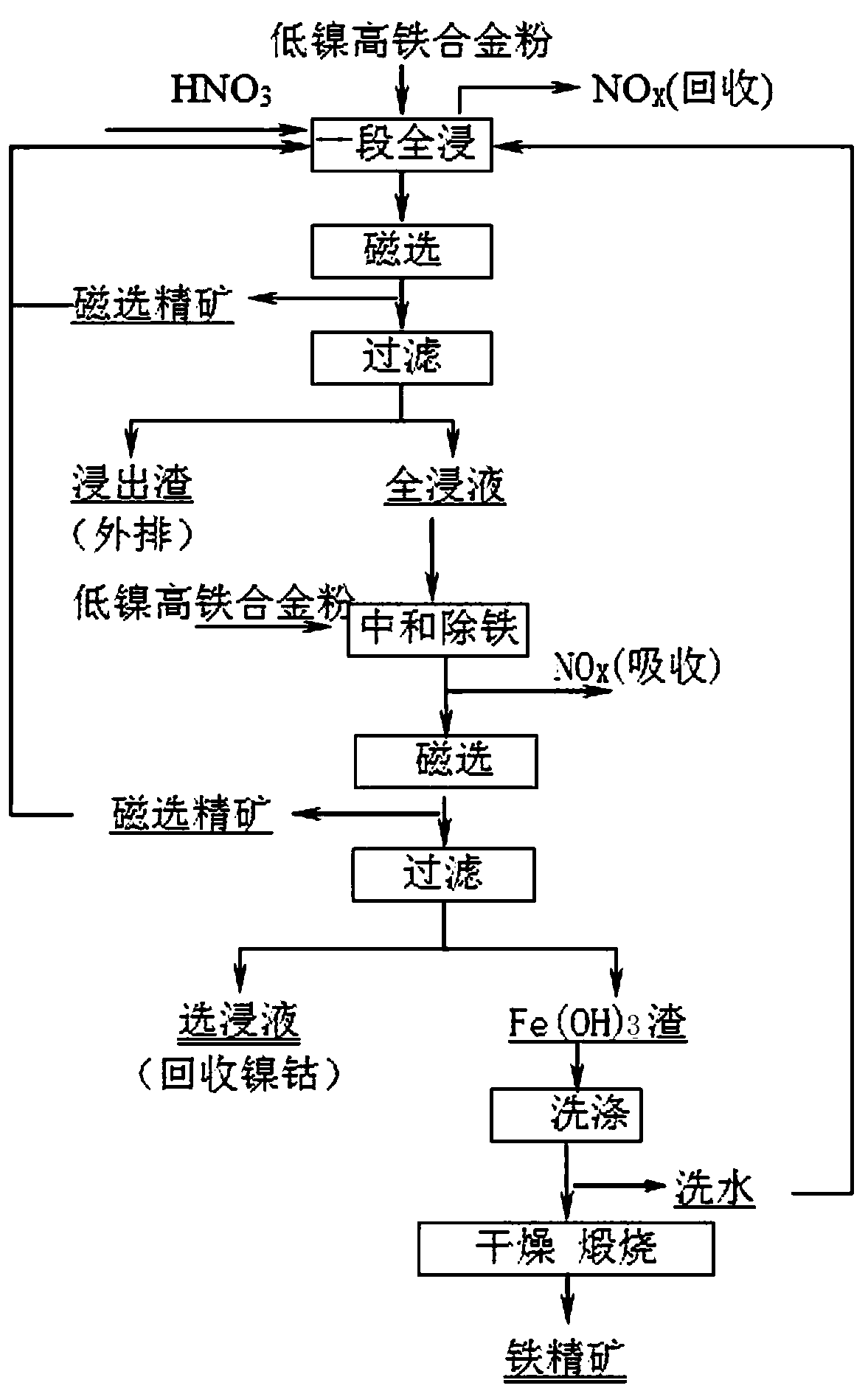
[0018] a) One-stage full immersion-magnetic separation: Add concentrated nitric acid to the low-nickel and high-iron alloy powder, the amount of concentrated nitric acid is 100ml / 100g of low-nickel and high-iron alloy powder, then add diluent to control the liquid-solid ratio to 5:1, the leaching temperature at 105°C, the leaching time is 4h, and NO is recovered during the leaching process x (a mixture of NO and NO2), magnetic separation is carried out at the end of full leaching, the magnetic separation concentrate returns to continue leaching, the rest of the pulp is filtered and separated from liquid to solid, and the obtained leaching slag is silicon slag, which is discharged outside;...
Embodiment 2
[0022] The composition of the low-nickel high-iron alloy powder is shown in Table 2.
[0023] Table 2: Composition of low-nickel and high-iron alloy powder (%)
[0024]
[0025] The nitric acid selective leaching method of the low-nickel high-iron alloy powder comprises the following steps:
[0026] a) One-stage full immersion-magnetic separation: Add concentrated nitric acid to the low-nickel and high-iron alloy powder, the amount of concentrated nitric acid is 200ml / 100g of low-nickel and high-iron alloy powder, then add diluent to control the liquid-solid ratio to 10:1, and the leaching temperature at 75°C, the leaching time is 8h, and NO is recovered during the leaching process x (a mixture of NO and NO2), magnetic separation is carried out at the end of full leaching, the magnetic separation concentrate returns to continue leaching, the rest of the pulp is filtered and separated from liquid to solid, and the obtained leaching slag is silicon slag, which is discharged ...
Embodiment 3
[0030] The composition of the low-nickel high-iron alloy powder is shown in Table 3.
[0031] Table 3: Composition of low-nickel and high-iron alloy powder (%)
[0032]
[0033] The nitric acid selective leaching method of the low-nickel high-iron alloy powder comprises the following steps:
[0034] a) One-stage full immersion-magnetic separation: Add concentrated nitric acid to the low-nickel and high-iron alloy powder, the amount of concentrated nitric acid added is 300ml / 100g of low-nickel and high-iron alloy powder, then add diluent to control the liquid-solid ratio to 8:1, and the leaching temperature at 90°C, the leaching time is 6h, and NO is recovered during the leaching process x (a mixture of NO and NO2), magnetic separation is carried out at the end of full leaching, the magnetic separation concentrate returns to continue leaching, the rest of the pulp is filtered and separated from liquid to solid, and the obtained leaching slag is silicon slag, which is discha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com