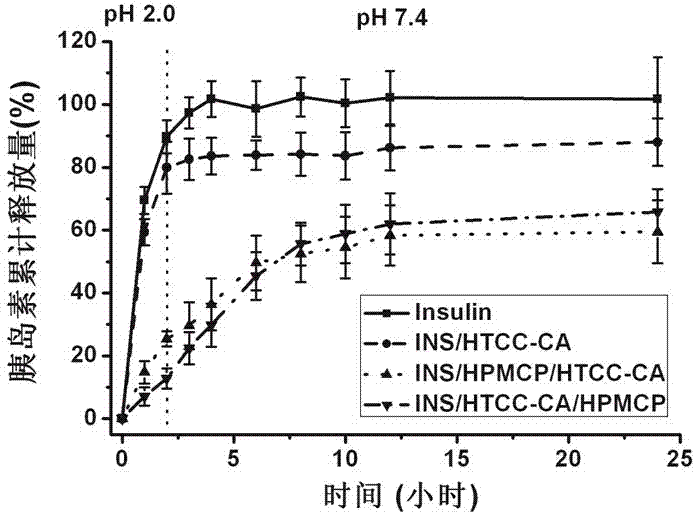
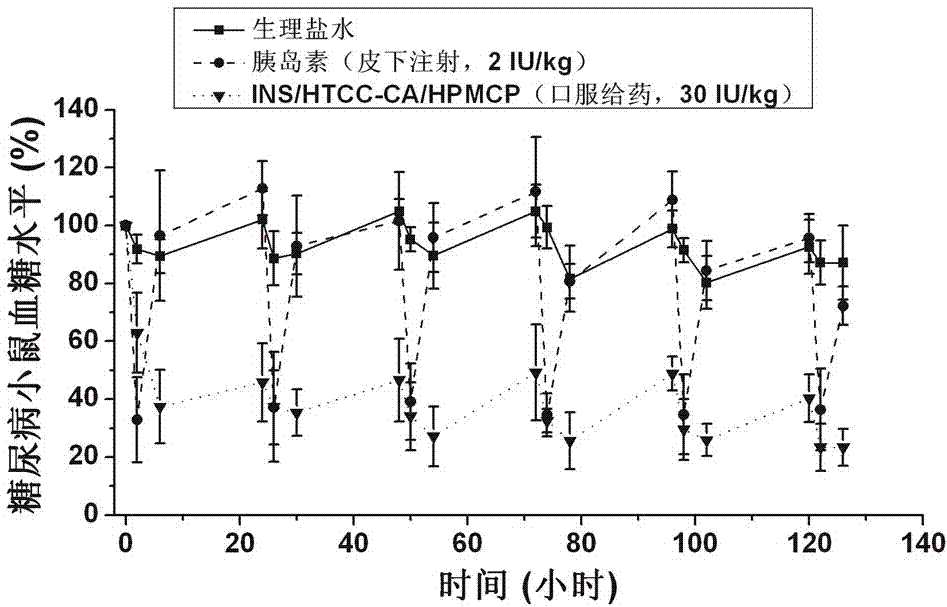
Insulin oral nano-preparation and preparation method thereof
A nano-formulation, insulin technology, applied in the field of medicine, can solve the problems of insulin inability to absorb and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
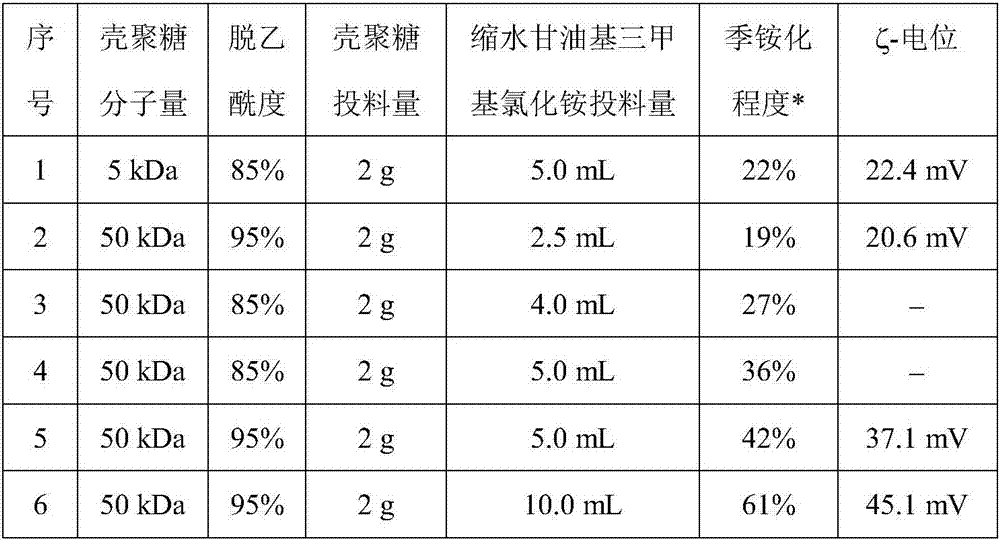
[0014] The preparation of embodiment 1. quaternized chitosan (HTCC)
[0015] Dissolve 2g chitosan (molecular weight 5-50kDa, deacetylation degree 85-95%) in 100mL aqueous solution containing 1% (v) acetic acid, add 50mL isopropanol and heat the solution to 60°C, dropwise After adding a certain volume of glycidyltrimethylammonium chloride, the solution was heated to 80° C. and stirred for 24 hours to carry out quaternization reaction. Precipitate the reaction product with acetone, then dissolve the precipitate in deionized water, repeat three times; then dissolve the precipitate in deionized water and dialyze against deionized water, and finally freeze-dry the solution to obtain a purified quaternized shell polysaccharides. with AgNO 3 Standard solution measures the chloride ion concentration in the quaternized chitosan solution, thereby calculates the degree of quaternization of chitosan, see table 1, the result shows under the situation of fixing chitosan charging amount, a...
Embodiment 2
[0019] Embodiment 2. Preparation of quaternized chitosan (HTCC-CA) modified by cholic acid
[0020] 0.5 g of quaternized chitosan with different degrees of quaternization was dissolved in 100 mL of deionized water. Dissolve 0.1-0.5g cholic acid (CA) in 50mL of anhydrous methanol, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) to the cholic acid methanol solution ( EDC) and N-hydroxysuccinimide (NHS), wherein cholic acid: EDC: NHS = 1: 1.5: 1.5 (molar ratio), after stirring at room temperature for 30 minutes, the methanol solution was slowly added dropwise to the quaternized shell The polysaccharide aqueous solution was stirred at 37° C. for 24 hours to react. The reaction product is firstly dialyzed against a weak base solution, then dialyzed against deionized water, and the dialyzed solution is freeze-dried to obtain purified cholic acid-modified quaternized chitosan. pass 1 H nuclear magnetic resonance spectrum calculates the degree of modification of ch...
Embodiment 3
[0026] Embodiment 3. Preparation of Insulin (INS) / HTCC-CA Nanoparticles - Blending Method
[0027] Dissolve insulin in 0.01mol / L HCl solution, adjust the pH of the solution to 7.4 to prepare 0.1-5.0 mg / mL insulin solution A. Cholic acid-modified quaternized chitosan (HTCC-CA) was dissolved in deionized water, and the pH of the solution was adjusted to 7.4 to prepare 0.1-20 mg / mL HTCC-CA solution B. A certain volume of insulin solution A was slowly added dropwise into a certain volume of HTCC-CA solution B and stirred to obtain INS / HTCC-CA nanoparticles 1 . The particle size of the nanoparticles was determined by light scattering (hydration diameter D h ) and ζ-potential. The unembedded free insulin in the solution was separated by ultrafiltration, the concentration of free insulin in the ultrafiltrate was measured by BCA kit, and the embedding efficiency of insulin was calculated by the following formula:
[0028] Embedding efficiency (%)=((total insulin mass-free insulin m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com