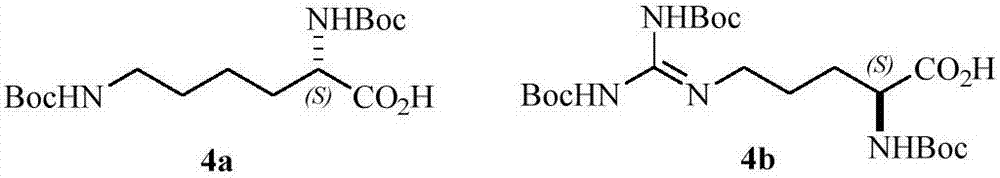Antibacterially active linezolid alkali cation amphiphilic compounds and synthesis method thereof
A technology for linezolid base cationic amphiphilic compounds, which is applied in the field of linezolid base cationic amphiphilic compounds and their preparation, can solve the problem that the antibacterial activity of the compound has not been significantly improved, and achieve broad-spectrum antibacterial activity, good Bacteriostatic effect, selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
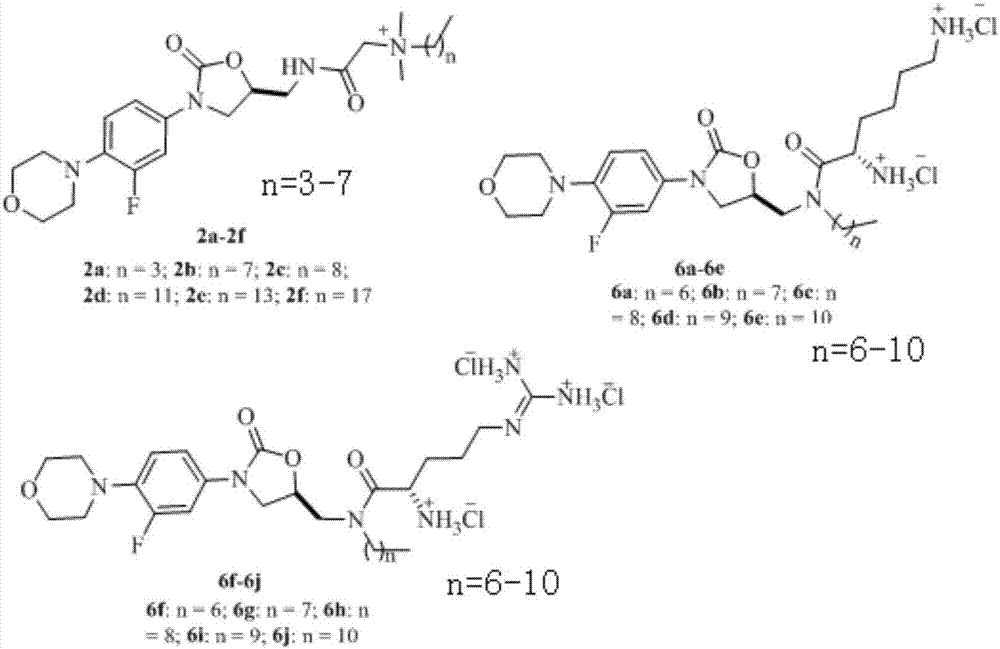
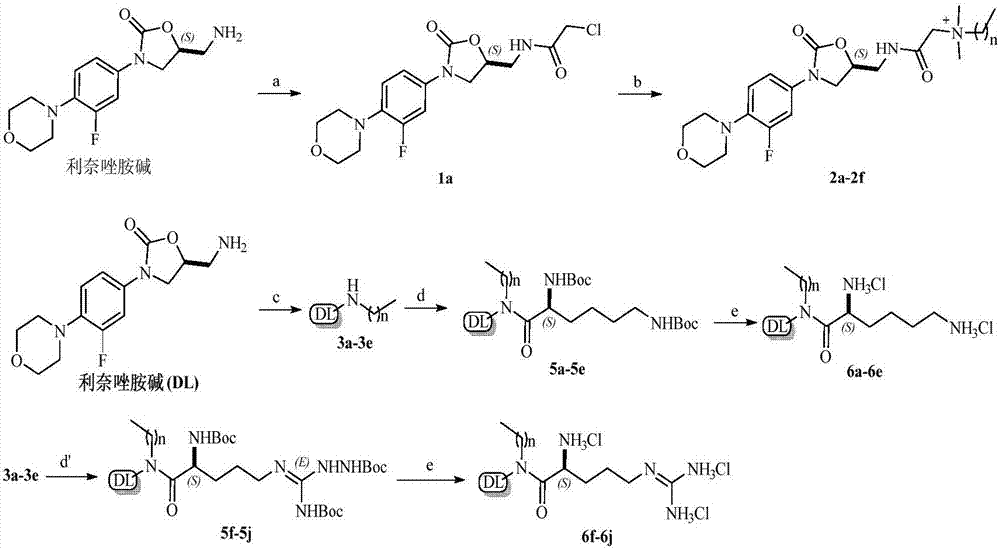
[0029] Example 1 Preparation of Compounds 2a~2f, 6a~6j
[0030] (1) Synthesis of compound (1a):
[0031] Synthesis of compound (1a): Add solvent acetone (25mL) to a flask containing a mixture of linezolid base (1g, 3.39mmol) and anhydrous potassium carbonate (562mg, 4.06mmol), then cover with a rubber stopper, and then use a syringe Chloroacetyl chloride (306 μL) was added, and the reaction was stirred at room temperature. After 0.5-1 h of reaction, the reaction was quenched with ice water, and a precipitate was precipitated, filtered with suction, washed with ice water, and the filter cake was dried in a vacuum oven to obtain the target product.
[0032] (2) Synthesis of compound (2a-2f):
[0033] Synthesis of compounds (2a-2f): Add solvent acetonitrile to the hydrothermal synthesis reactor containing compound 1a, and then add N,N-dimethyl n-alkylamine (1a: N,N-dimethyl n-alkyl Amine=1:3, molar ratio), and then stirred and reacted in an oil bath at 85°C. After reacting for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com