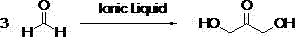Method for preparing 1,3-dihydroxyacetone through formaldehyde condensation reaction catalyzed by ionic liquid
A technology of dihydroxyacetone and ionic liquid, which is used in the preparation of carbonyl compounds by condensation, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of complicated separation process, expensive catalyst and high separation cost, and achieves simple and easy reaction process. Operation and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
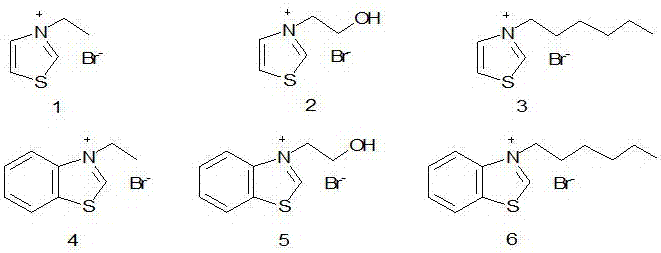
[0039] Add 4.5g of paraformaldehyde, 1.7g of ionic liquid 1, 0.8g of triethylamine, 60mL of ethanol in a 100mL reaction kettle, stir mechanically, and heat up to 90°C within 30min. o C, reacted for 3h under the condition of nitrogen protection. Cool after the reaction, remove the catalyst by filtration, analyze the reaction solution by gas chromatography, and quantify with the external standard method. The yield of dihydroxyacetone is 41.7%.
Embodiment 2
[0041] Same as Example 1, using ionic liquid 4 as the catalyst, the feeding amount is 1.8g, and the yield of dihydroxyacetone is 50.1%.
Embodiment 3
[0043] Same as Example 1, using ionic liquid 6 as the catalyst, the feeding amount is 2.04g, and the yield of dihydroxyacetone is 52.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com