Photoinitiator and preparation method thereof
A technology of photoinitiator and carbon atom, which is applied in the field of photoinitiator and its preparation, can solve the problems of low absorption capacity, poor performance of photoinitiator, and low efficiency of photosensitive free radicals, etc., to achieve strong absorption and high efficiency against the surface The effect of oxygen inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
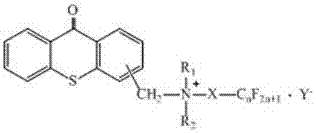
[0039] A photoinitiator whose structure is shown in formula (M):
[0040]
[0041] (M)
[0042] Wherein, the substitution position on the thioxanthone aromatic ring is 2 and / or 4; R 1 and R 2 An alkyl group of 1 to 7 carbon atoms or a combination of the two into an n-alkylene group of 4 to 5 carbon atoms; X is —Z-S-CH 2 CH 2 —, where Z is an alkylene group with 2 to 6 carbon atoms, and the Z group is connected to the nitrogen atom of the quaternary ammonium salt; n in the fluorocarbon chain is 6 to 8; Y - is a nucleophilic anion.
[0043] Preferably, the Y- for PF 6 ¯, SbF 6 ¯ or Ph 4 B.
[0044] Preferably, the Y - for Ph 4 B.
Embodiment 2
[0046] The preparation method of above-mentioned photoinitiator, comprises the following steps:
[0047] S 1 . Preparation of fluorocarbon chain quaternary ammonium salt:
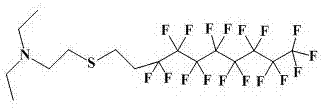
[0048] Using 20ml tetrahydrofuran as the medium, add 2g perfluorooctyl bromide, 0.73g N,N-diethylethanethiolamine, heat the reaction system to 60°C, add 0.3 g sodium hydroxide, perfluorooctyl The molar ratio of ethyl bromide, N,N-diethylethanethiolamine, and sodium hydroxide is 1.0:1.5~5:1.5~5, and the reaction system is reacted at 60°C for 48h; extracted with water, and the solvent is spin-dried , obtain 1.7g off-white liquid, productive rate 84.16%, product B 1 The structure of diethylaminoethyl-perfluorooctyl ethyl sulfide is as figure 2 As shown, the NMR data are: 1 H NMR (400 MHz, CDCl 3 ): δ2.71 (dd, J = 18.0, 9.4 Hz, 1H),2.66–2.54 (m, 2H), 2.50 (q, J = 7.1 Hz, 2H), 2.42-2.26 (m, 1H), 0.98 (t, J =7.1 Hz, 3H).
[0049] S 2 . Preparation of thioxanthone modified by fluorocarbon chain quate...
Embodiment 3
[0055] A kind of preparation method of photoinitiator, comprises the following steps:
[0056] S1. Preparation of fluorocarbon chain quaternary ammonium salt:
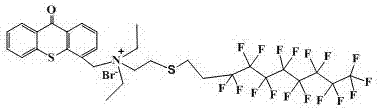
[0057] With 20ml tetrahydrofuran as the medium, add 2g perfluorooctyl bromide, 0.70g N,N-dimethylethanethiolamine, heat the reaction system to 60°C, add 0.3 g sodium hydroxide, perfluorooctyl The molar ratio of ethyl bromide, N,N-diethylethanethiolamine, and sodium hydroxide is 1.0:1.5~5:1.5~5, and the reaction system is reacted at 60°C for 48h; extracted with water, and the solvent is spin-dried , to obtain slightly beige liquid, yield 77.08%, product B 2 The structure of N,N-dimethylethylammonia-perfluorooctylethylsulfide is as follows Figure 5 As shown, the NMR data are 1 H NMR (400 MHz, CDCl 3 ) δ2.79 (t, J = 16.1, 8.2 Hz, 2H),2.73–2.64 (t, 2H), 2.53 (t, J = 14.8, 8.1 Hz, 2H), 2.40 (m, J = 30.9, 19.6,11.2 Hz, 2H), 2.33 (s, J = 38.0 Hz, 6H).
[0058] S 2 . Preparation of thioxanthone modified by fluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com