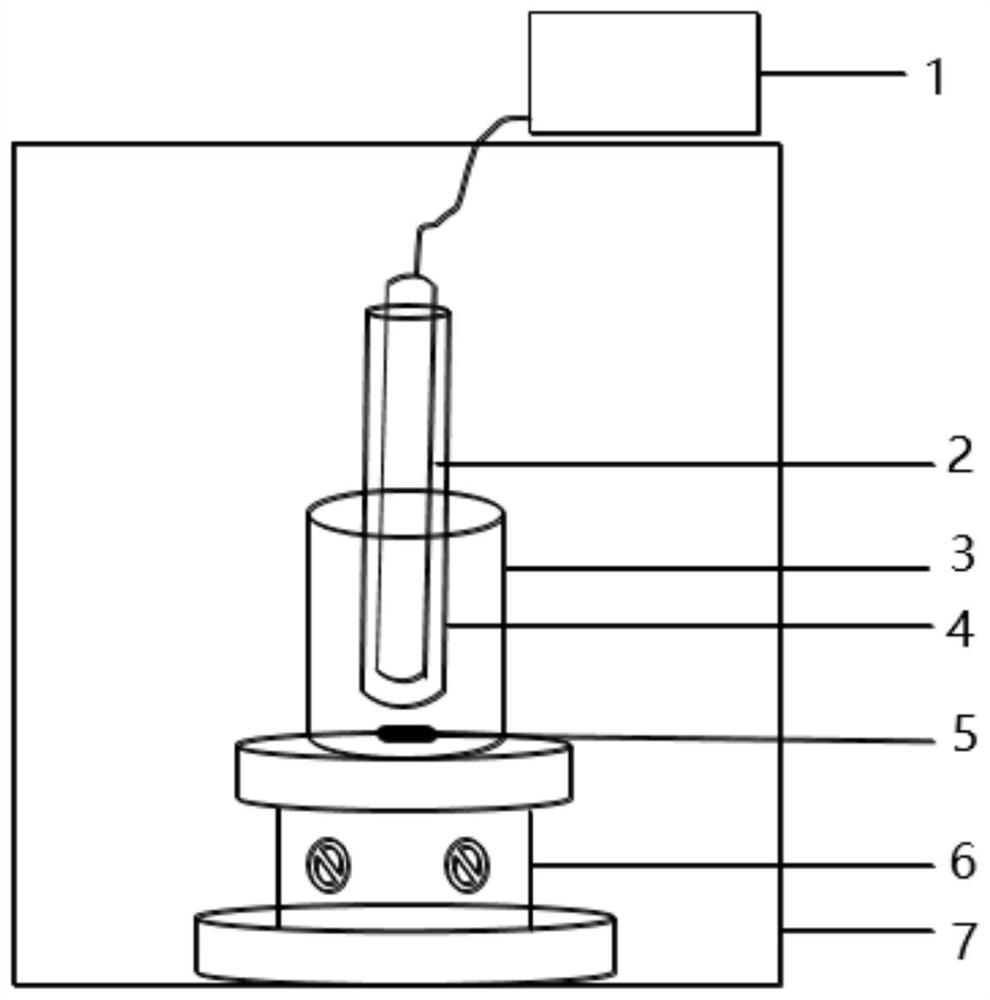
A method for photocatalytic degradation of perfluorinated compounds
A technology of perfluorinated compounds and photocatalysis, applied in chemical instruments and methods, water treatment of special compounds, water pollutants, etc., can solve problems such as high operating costs, high energy consumption, and inability to defluorinate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 5.22g of CTAB in 208mL of deionized water, stir at a medium speed until CTAB is completely dissolved, slowly add 16mLTEOS, then quickly add 4.8mL of 25% ammonia water, continue stirring until the solution turns into a white gel, filter and dry. The base material BMMs were obtained by heating from room temperature to 550°C for 6 hours at a heating rate of 5°C / min.
[0036] Dissolve 0.10g~0.50g (0.10g, 0.20g, 0.40g, 0.50g) of phosphotungstic acid in 10mL of deionized water respectively, add 1.00g of BMMs after the dissolution is complete, adjust the pH to 0.90 with nitric acid, stir for 12 hours, 80 The solvent was evaporated to dryness at 110°C for 12 hours, and the grinding was complete. Under a nitrogen atmosphere, the temperature was raised from room temperature to 200°C at a rate of 5°C / min and calcined for 2 hours to obtain the catalyst BMMs / HPW.
[0037] In this embodiment, the catalyst BMMs / HPW with a loading rate of phosphotungstic acid of 10% to 50% (10...
Embodiment 2
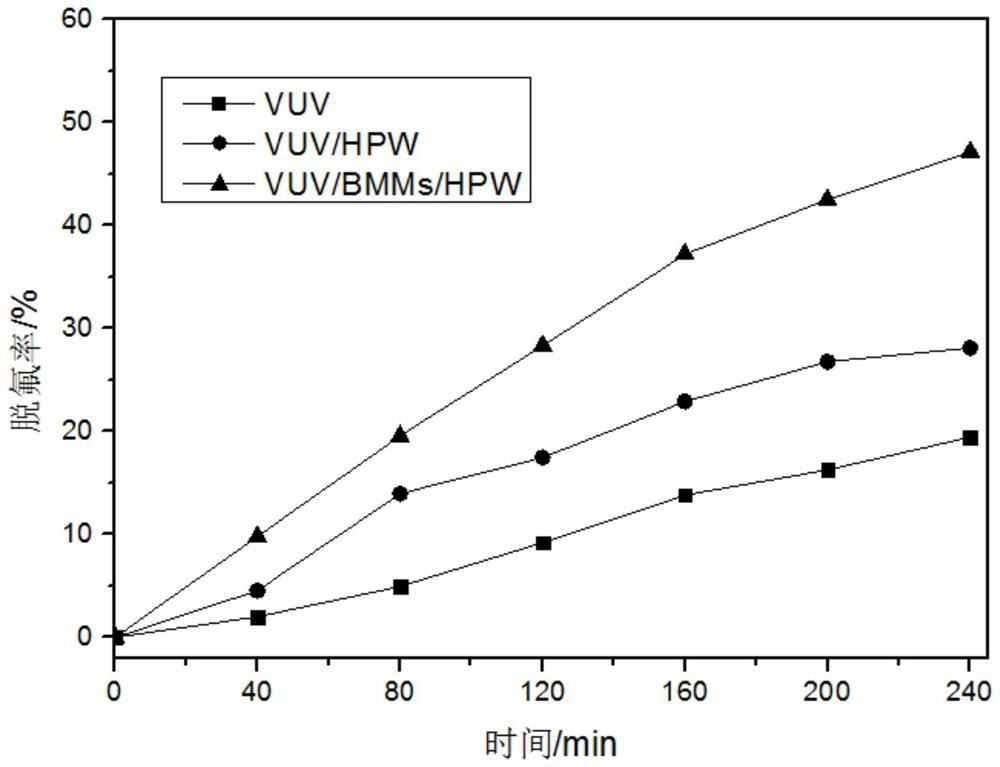
[0039] Put 500mL of perfluorooctanoic acid (PFOA) solution containing 10mg / L in a cylindrical reactor, without adjusting the pH, set up 3 groups of comparative experiments, the first group did not add catalyst, the second group added 0.10g phosphotungstic acid, and the third group Add 0.10g catalyst BMMs / HPW with a loading rate of 40% to the group, cover a low-pressure mercury lamp with an emission wavelength of 185nm with a glass sleeve, place it vertically in the reactor, and place the reactor on a constant temperature magnetic stirrer. When the ultraviolet lamp is in use, the compartment outside the reactor is closed to start defluorination, and a sample is taken every 40 minutes. After 4 hours, the reaction was completed, and the fluoride ion concentration in the water was measured by ion chromatography, and the defluorination effect was shown in image 3 .
[0040] F in initial solution of perfluorooctanoic acid (PFOA) - The concentration is about 0 (not detected), no p...
Embodiment 3
[0044] Put 500mL of perfluorooctanoic acid (PFOA) solution containing 10mg / L in a cylindrical reactor, do not adjust the pH, and add 0.10g each with a loading rate of 10% to 50% (10%, 20%, 40%, 50%) For BMMs / HPW, a low-pressure mercury lamp with an emission wavelength of 185nm is covered with a glass sleeve, placed vertically in the reactor, and the reactor is placed on a constant temperature magnetic stirrer. When the ultraviolet lamp is in use, the compartment outside the reactor is closed to start defluorination, and a sample is taken every 40 minutes. After 4 hours, the reaction was completed, and the fluoride ion concentration in the water was measured by ion chromatography, and the defluorination effect was shown in Figure 4 .
[0045] F in initial solution of perfluorooctanoic acid (PFOA) - The concentration is about 0 (not detected), no pretreatment is required, and it can be directly degraded.
[0046] In this example, the BMMs / HPW synthesized in Example 1 was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| load ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com