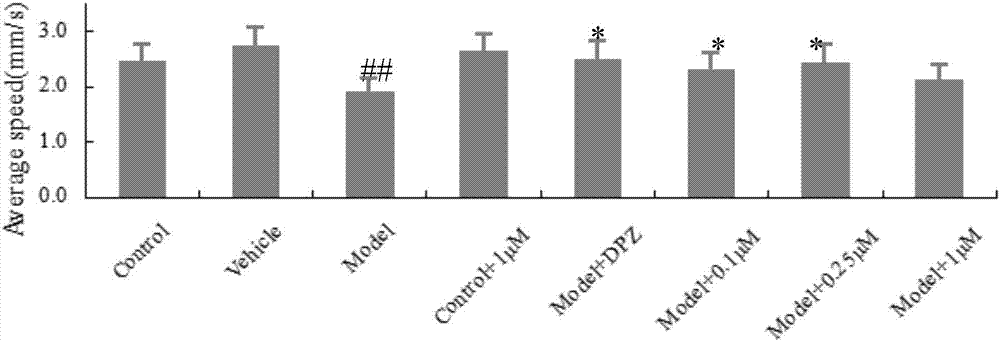
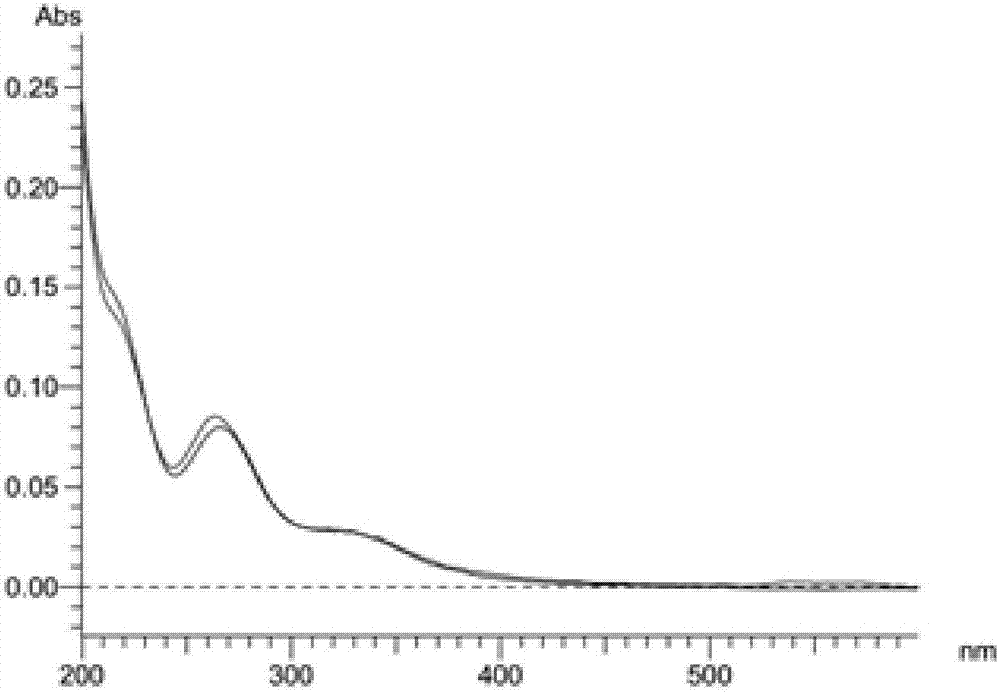
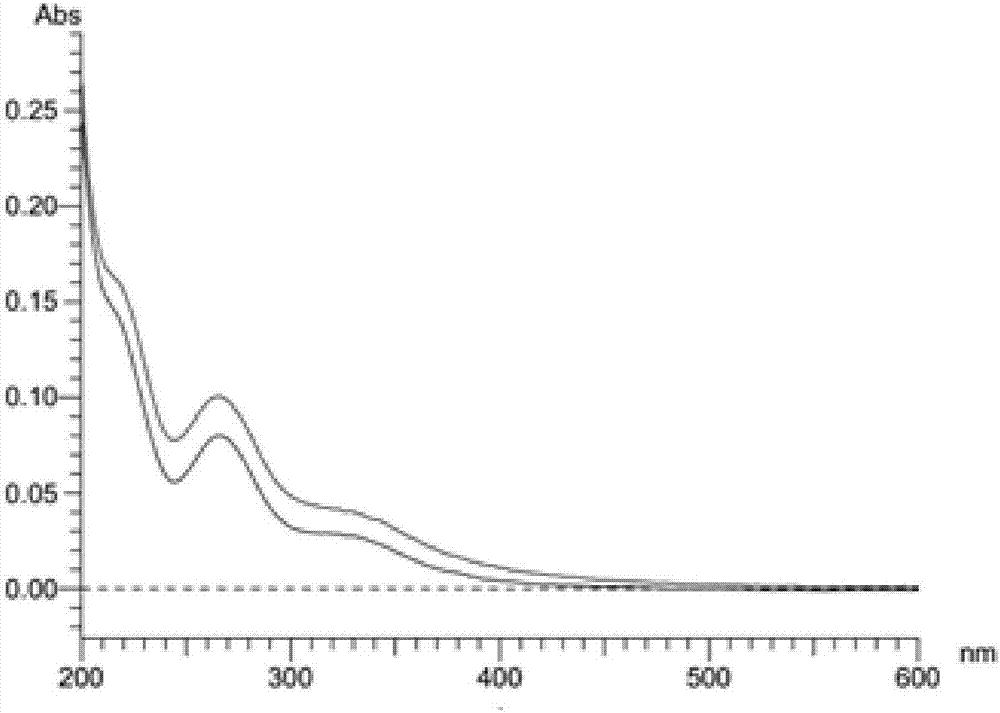
2,4-bis-substituted acetophenone compound, optical isomers and pharmaceutically acceptable salts thereof and application of 2,4-bis-substituted acetophenone compound and optical isomers and pharmaceutically acceptable salts thereof
A technology of optical isomers and acetophenone, applied in the field of derivatives of acetophenone, can solve the problems of complex pathogenesis, inability to effectively control or cure AD, and difficult to contain or reverse the development of AD disease.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1: the preparation of 3-(1-hydroxyl) ethylphenol (IIIa)
[0089] With 150.00g (1.10mol) 3-hydroxyacetophenone compound (corresponding to R in formula II 3 (for methyl) was dissolved in 500ml of tetrahydrofuran, and 51.00g (1.30mol) of sodium borohydride was added in batches in an ice bath, stirred at room temperature, and the reaction progress was monitored by TLC. After the reaction, tetrahydrofuran was removed by rotary evaporation, sodium borohydride was quenched with ice water, and dilute hydrochloric acid was added dropwise until the pH value reached 5-6. The aqueous layer was extracted three times with ethyl acetate, and the combined ethyl acetate layer was dried over anhydrous sodium sulfate, then concentrated and evaporated to dryness to obtain a pale yellow solid IIIa with a yield of 83.1%.
[0090] 1 H-NMR (δ, CDCl 3 ):7.23(s,1H,-OH),7.08(dd,1H,J 1 =J 2 =8.0Hz, ArH), 6.77(s,1H,ArH),6.73(d,1H,J=8.0Hz,ArH),6.59(dd,1H,J 1 =8.0 Hz,J 2 =1.6Hz, ArH...
Embodiment 2
[0091] Embodiment 2: the preparation of 3-(1-hydroxyl) ethyl ethyl benzoate (IVa)
[0092] Dissolve 126.60g (0.90mol) of compound IIIa in 500ml of potassium hydroxide solution with a concentration of 5.50mol / L at 0°C, add 113ml (1.17mol) of acetic anhydride dropwise with stirring at 0°C, complete the dropwise addition within half an hour and store at room temperature Stir for 2h. After the reaction, the reaction system was extracted three times with ethyl acetate, and the mixed ethyl acetate layer was extracted three times with saturated sodium bicarbonate, saturated sodium chloride and water, respectively, and then dried with anhydrous sodium sulfate, concentrated and evaporated to dryness. Yellow liquid IVa, yield 76.3%.
[0093] [M+H] + =181.
Embodiment 3
[0094] Embodiment 3: the preparation of 3-(1-chloro) ethyl ethyl benzoate (Va)
[0095] 125.50g (0.70mol) of compound IVa was dissolved in 500ml redistilled dichloromethane and 123ml (1.61mol) redistilled N,N-dimethylformamide (DMF) mixed solution, and 67ml ( 1.05mol) redistilled thionyl chloride and stirred for 2h. After the reaction, slowly add saturated sodium carbonate solution dropwise at 0°C to adjust the pH value of the reaction system to 7-8. Afterwards, it was extracted three times with ethyl acetate, and the mixed ethyl acetate layer was dried over anhydrous sodium sulfate, concentrated and evaporated to dryness to obtain Va, a brownish-yellow liquid, with a yield of 91.8%.
[0096] [M+H] + =199.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com