A kind of quinoline-substituted coumarin derivative, its preparation method and its application in ratio-type pH fluorescent probe
A technology of coumarin derivatives and fluorescent probes, which is applied in the field of analytical chemistry, can solve the problems of external environment, probe load and retention, etc. , accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of embodiment 1pH fluorescent probe 3-[2-[8-carboxyquinolin-2-yl]vinyl]-7-diethylaminocoumarin
[0026] This embodiment provides a synthetic method for 3-[2-[8-carboxyquinolin-2-yl]vinyl]-7-diethylaminocoumarin, the main steps of which are as follows:
[0027] Add successively 0.3745g (2.0026mmoS) 2-methyl-8-carboxyquinoline, 0.5904g (2.4000mmoS), 2.00mL acetic acid, 0.6991g sodium acetate, 2.88mL acetic anhydride in the 50mL two-necked bottle, stir and reflux for 6 hours, After cooling, a red solid precipitated out, which was filtered by suction and washed with ethanol to obtain 0.6657 g of the product, with a yield of 80.40%. 1 H NMR (400MHz, CDCS 3 )δ8.72 (m, J=7.3,1.4Hz,1H),8.29(d,J=8.7Hz,1H),8.01(m,J=8.1,1.3Hz,1H),7.86(s,1H), 7.80(d,J=16.1Hz,1H),7.72(d,J=8.7Hz,1H),7.67–7.62(m,1H),7.61(d,J=2.7Hz,1H),7.36(d,J =8.9Hz, 1H), 6.63(m, J=8.9, 2.4Hz, 1H), 6.48(d, J=2.2Hz, 1H), 3.45(dd, J=7.1Hz, 4H), 1.70(s, 1H ),1.24(t,J=7.1Hz,6H).
[0028] image 3 For the ...
Embodiment 2
[0030] The preparation of embodiment 2 test solution
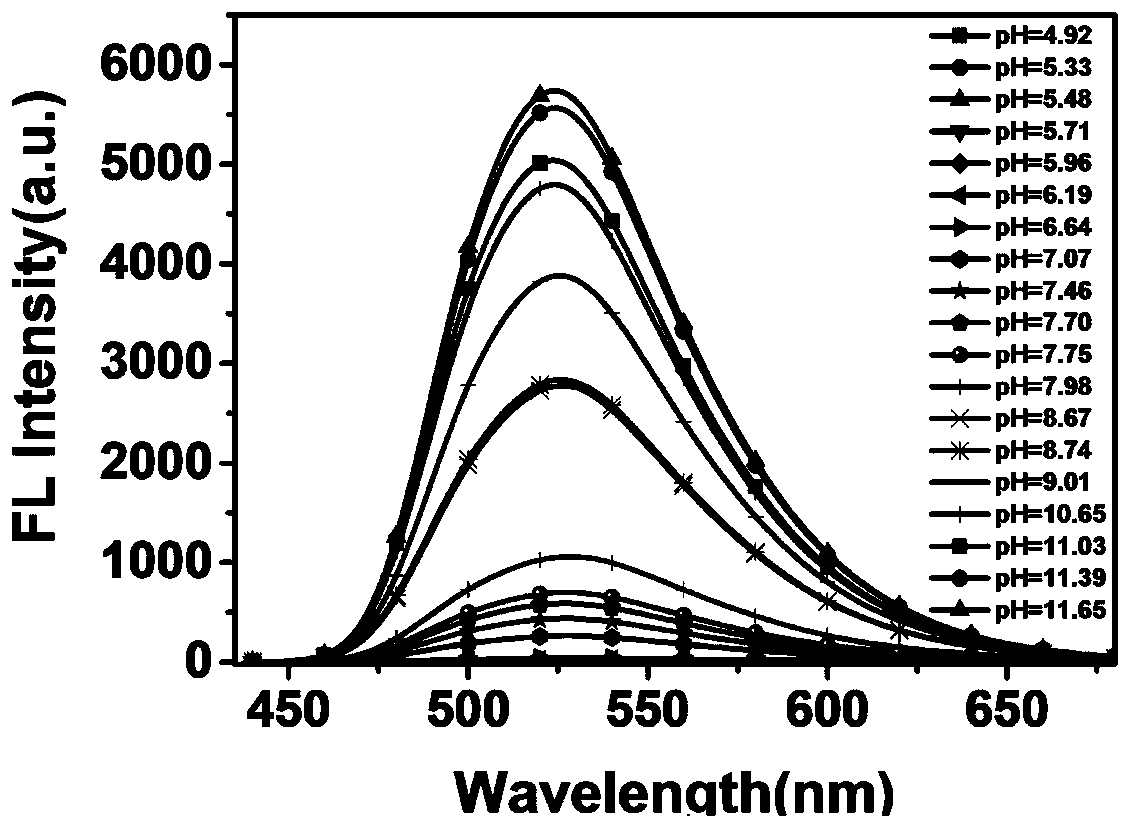
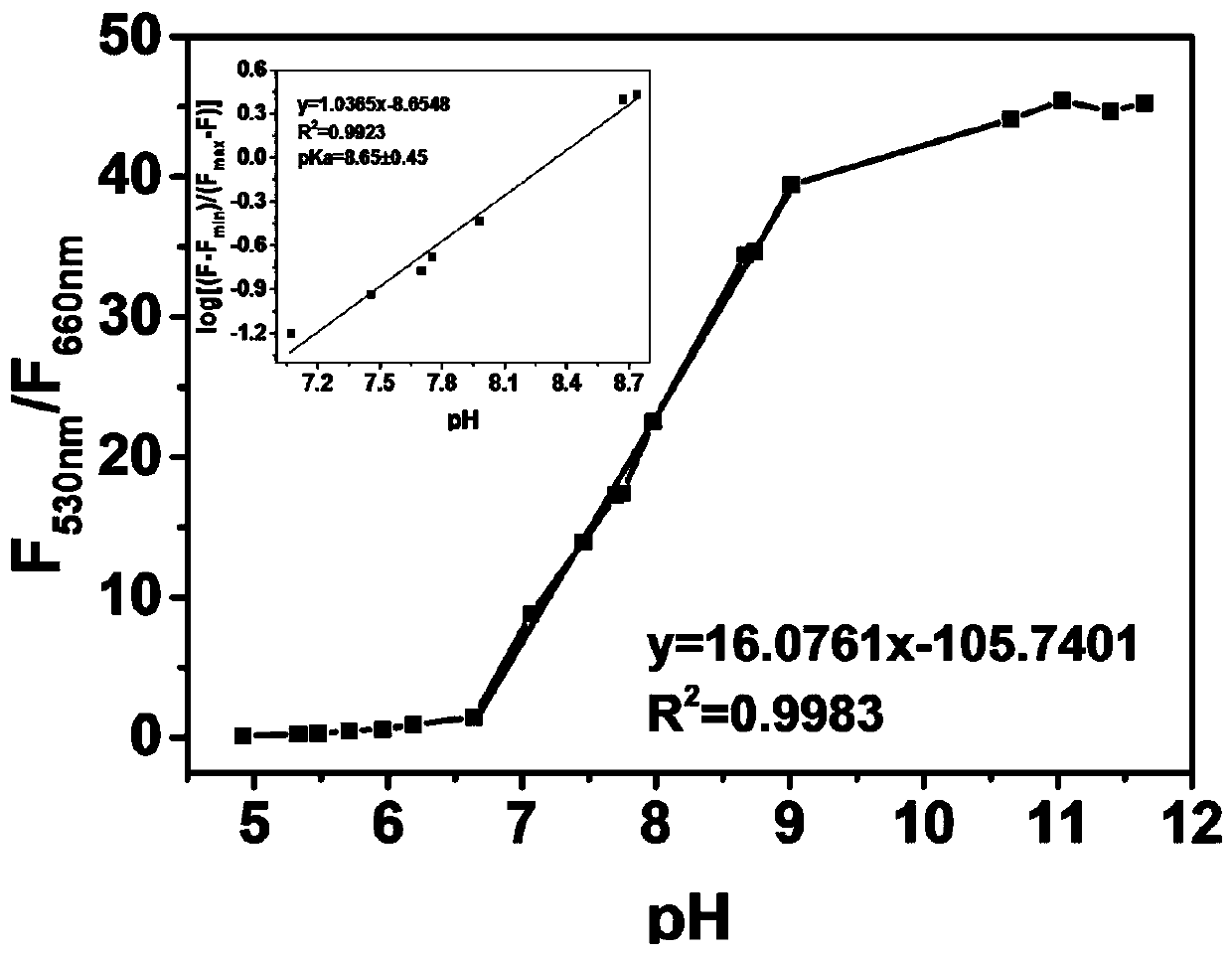
[0031] (1) The preparation procedure of the stock solution: in a 10mL sample bottle, prepare 10 mL of the stock solution with chloroform -3 mol / L stock solution for use. Prepare V (methanol): V (PBS buffer solution) = 9:1 with different pH values (4.92, 5.33, 5.48, 5.71, 5.96, 6.19, 6.64, 7.07, 7.46, 7.70, 7.75, 7.98, 8.67, 8.74, 9.01 , 10.65, 11.03, 11.39, 11.65) of the buffer solution was monitored with a magnetic pH meter.
[0032] (2) Test of the fluorescence emission spectrum of the probe against pH changes: take 2 mL of different pH values (4.92, 5.33, 5.48, 5.71, 5.96, 6.19, 6.64, 7.07, 7.46, 7.70, 7.75, 7.98, 8.67, 8.74, 9.01 , 10.65, 11.03, 11.39, 11.65) buffer solution, adding 20 μL of the stock solution configured in Example 2 (1) for fluorescence spectrum testing.
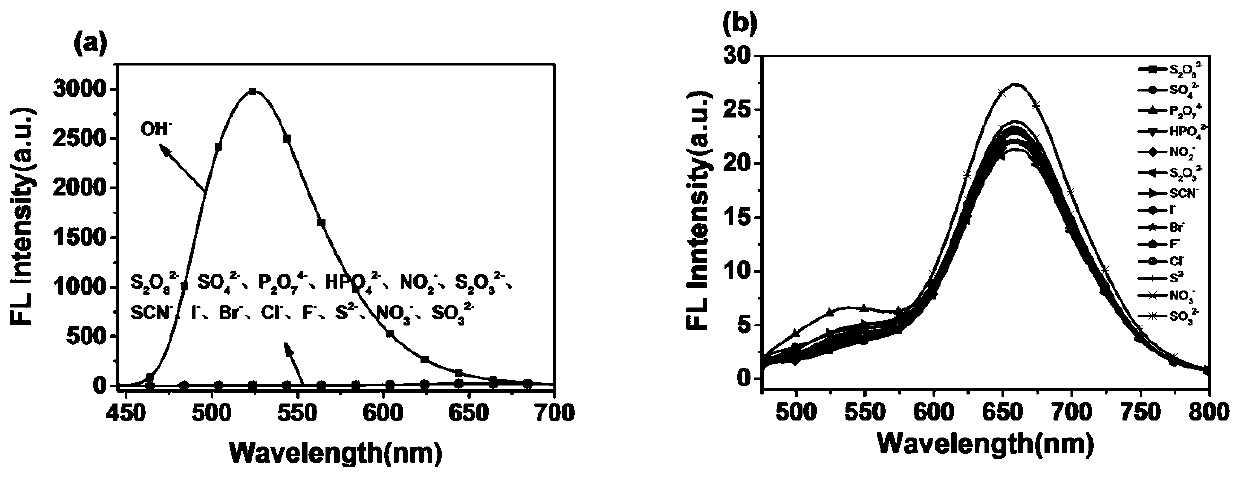
[0033] (3) Probe S to OH -Selectivity experiment: get 14 parts of 2mL pH=3.15 buffer solution, each add 20 μL of the stock solution prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com