A kind of organosilicon benzoxazine resin prepolymer based on eugenol and its preparation method and application
A technology of silicon benzoxazine and organosilicon compound, which is applied in the field of organosilicon benzoxazine resin prepolymer and its preparation, and achieves the effects of simple operation, simple preparation and good corrosion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
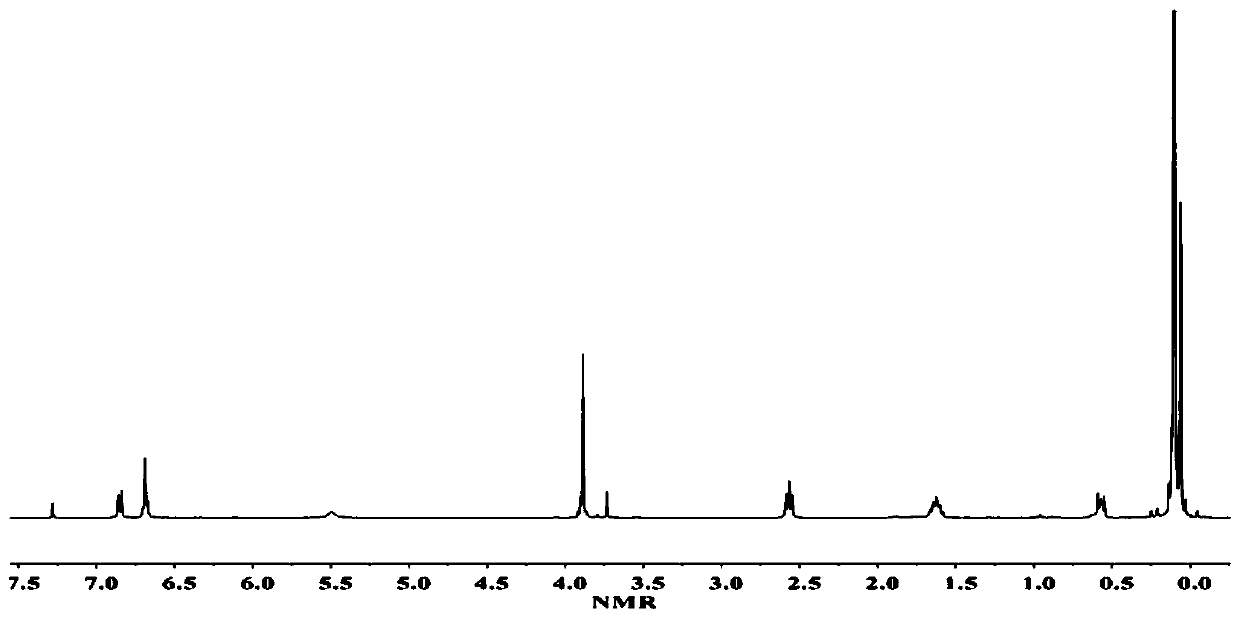
[0049] (1) Dissolve 0.5mol eugenol, 0.2mol 1,1,3,3-tetramethyldisiloxane, and 0.01mol Karstedt catalyst in a mixed solution of 500mL dioxane and toluene at 70°C The reaction was carried out under reduced pressure for 32 hours, the mixed solvent was removed by rotary evaporation under reduced pressure, washed with water and dried to obtain bis-eugenol-1,1,3,3-tetramethyldisiloxane, the structure of which was shown in formula (1-1), Yield was 95.5%, H NMR spectrum 1 H-NMR such as figure 1 As shown, each peak on the figure is in one-to-one correspondence with the hydrogen atoms on the structure of the biseugenol-1,1,3,3-tetramethyldisiloxane compound.
[0050]
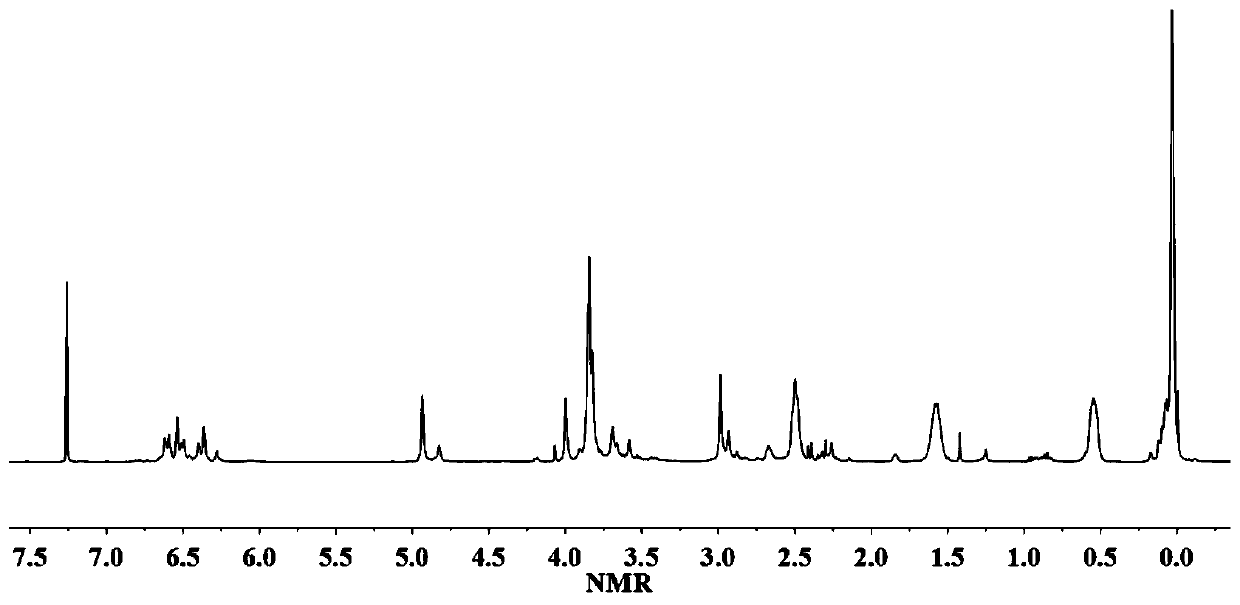
[0051] (2) Dissolve 0.1mol of the dieugenol-1,1,3,3-tetramethyldisiloxane compound prepared above in 180mL of toluene solution, add 0.11mol of ethylenediamine and 0.6mol of paraformaldehyde at 0°C After reacting for 66 hours, the mixed solvent was distilled off under reduced pressure, and dried to obtain bis-eugenol-...
Embodiment 2
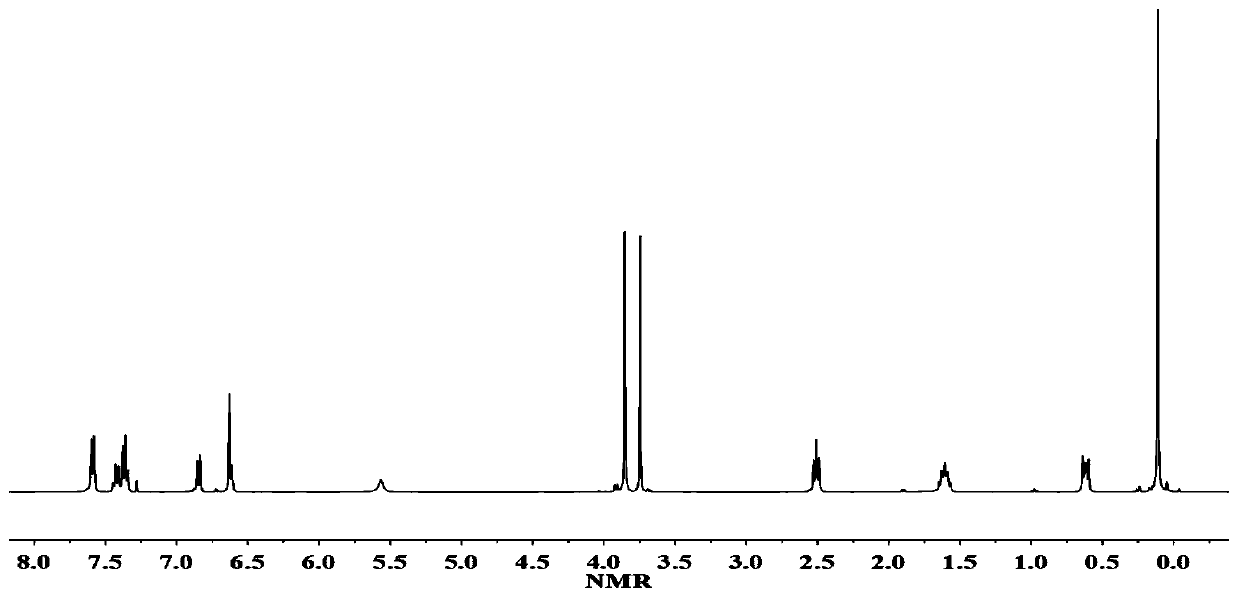
[0055] (1) Dissolve 0.5mol eugenol, 0.15mol 1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane, 0.02mol Speier catalyst in 500mL dioxane and toluene In the mixed solution, react at 30°C for 22 hours, remove the mixed solvent by rotary evaporation under reduced pressure, wash with water and dry to obtain biseugenol-1,1,5,5-tetramethyl-3,3-diphenyltris Siloxane, its structure is shown in formula (1-3), and productive rate is 97.1%, and hydrogen nuclear magnetic resonance spectrum 1 H-NMR such as image 3 As shown, there is a one-to-one correspondence between each peak on the figure and the hydrogen atoms on the structure of biseugenol-1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane compound.
[0056]
[0057] (2) After dissolving 0.1mol of the dieugenol-1,1,5,5-tetramethyl-3,3-diphenyltrisiloxane compound prepared above in a mixed solution of 210mL dioxane and toluene Add 0.11mol ethylenediamine and 0.57mol paraformaldehyde, react at 15°C for 18 hours, distill off the mixed solvent un...
Embodiment 3
[0061] (1) Dissolve 0.5mol eugenol, 0.17mol 1,1,3,3-tetraisopropyldisiloxane, 0.01mol Speier catalyst in a mixed solution of 500mL dioxane and toluene, at 40°C After reacting for 31 hours, the mixed solvent was removed by rotary evaporation under reduced pressure, washed with water and dried to obtain bis-eugenol-1,1,3,3-tetraisopropyldisiloxane compound with a yield of 89%.
[0062] (2) Dissolve 0.1mol of the dieugenol-1,1,3,3-tetraisopropyldisiloxane compound prepared above in 250mL of dioxane solution and add 0.1mol of propylenediamine and 0.53mol of poly Formaldehyde, reacted at 30°C for 26 hours, distilled off the mixed solvent under reduced pressure, and dried to obtain bis-eugenol-1,1,3,3-tetraisopropyldisiloxane-propylenediamine silicone benzoxazine prepolymer , whose structure is shown in formula (1-5), and the yield is 98%.
[0063]
[0064] The biseugenol-1,1,3,3-tetraisopropyldisiloxane-propylenediamine silicone benzoxazine prepolymer was UV-cured with a 500W b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com