Method for producing glyoxylate through oxidation of glycollate
A technology of glycolic acid ester and glyoxylic acid ester, applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc., can solve the problems of low yield and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
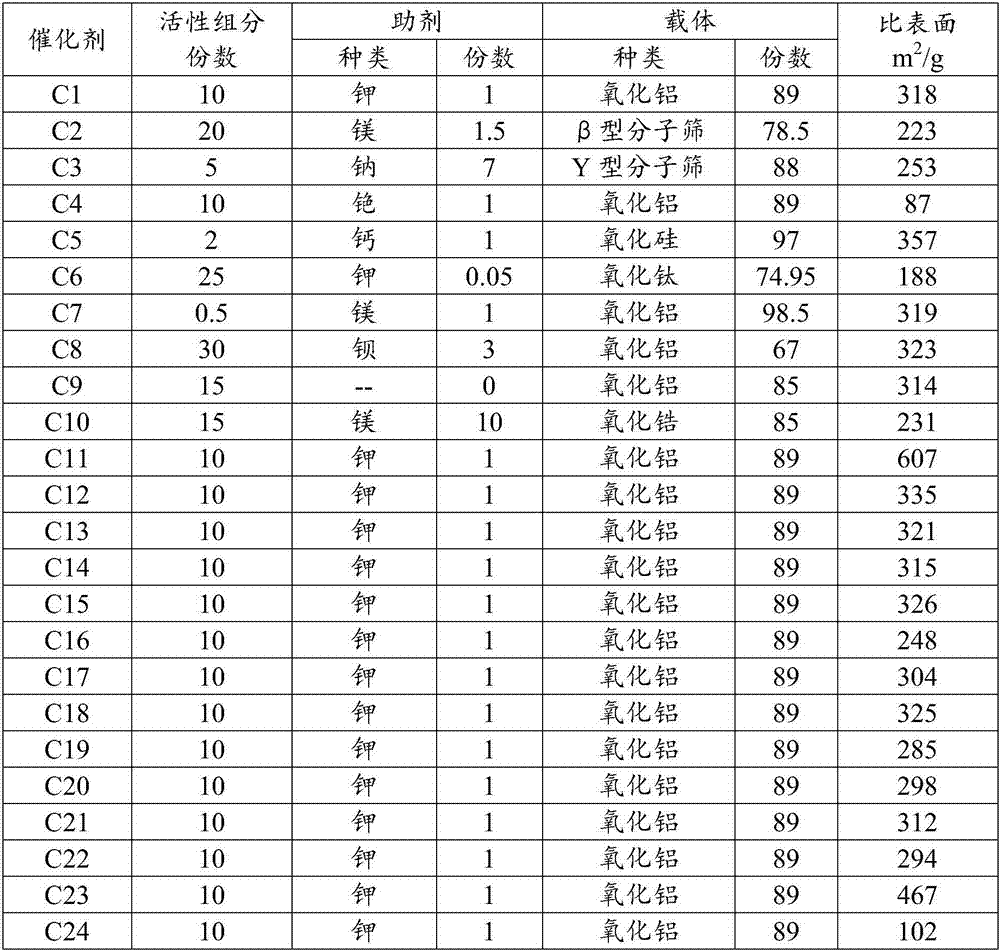
[0031] Prepare 200ml of ferric nitrate solution I containing 5.6% iron and stir, and the specific surface of 100g is 305m 2 / g alumina carrier was added to solution I and stirred evenly to obtain slurry II, and then ammonia water was added dropwise to slurry II under stirring, and the pH at the end point was controlled to be 8 to obtain slurry IV, which was aged at 80°C for 5 hours and then taken out The product was filtered, and the filter cake was washed with ethanol and deionized water, and dried at 120°C. Then prepare 50ml of potassium nitrate solution containing 2.3% potassium, impregnate and dry the filter cake, dry at 120°C, and roast at 500°C to obtain catalyst C1, take catalyst samples for X-fluorescence (XRF) determination of catalyst components, and specific surface BET characterization The test results are shown in Table 1.
Embodiment 2
[0033] Prepare 200ml solution I of ferric nitrate containing 12.8% iron according to the method of [Example 1] and stir, and the specific surface of 100g is 208m 2 / gβ-type molecular sieve carrier was added to solution I and stirred evenly to obtain slurry II, and then ammonia water was added dropwise to slurry II under stirring, and the pH at the end point was controlled to be 8 to obtain slurry IV, which was aged at 80°C for 5 hours and then taken out The product was filtered, and the filter cake was washed with ethanol and deionized water, and dried at 120°C. Then prepare 40ml of magnesium nitrate solution containing 4.8% magnesium, impregnate and dry the filter cake, dry at 120°C, and roast at 500°C to obtain catalyst C2. The catalyst samples are taken for cesium XRF and BET characterization tests. The test results are shown in Table 1.
Embodiment 3
[0035] According to the method of [Example 1], 200ml of solution I of iron nitrate containing 2.9% iron was prepared and stirred, and the specific surface of 100g was 325m 2 / g Y-type molecular sieve carrier was added to solution I and stirred evenly to obtain slurry II, and then ammonia water was added dropwise to slurry II under stirring, and the pH at the end point was controlled to be 8 to obtain slurry IV, which was aged at 80°C for 5 hours The product was taken out and filtered, and the filter cake was washed with ethanol and deionized water, and dried at 120°C. Then prepare 50ml of sodium nitrate solution containing 15.9% sodium, impregnate and dry the filter cake, dry at 120°C, and roast at 500°C to obtain catalyst C3. The catalyst samples were taken for XRF and BET characterization tests. The test results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com