Method for producing ethylene glycol from formaldehyde
A technology of ethylene glycol and formaldehyde, which is applied in the production of bulk chemicals, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as low yields of glycolic acid and ethylene glycol, harsh reaction conditions, and long process flow , to achieve the effect of increasing yield, reducing pressure and shortening the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
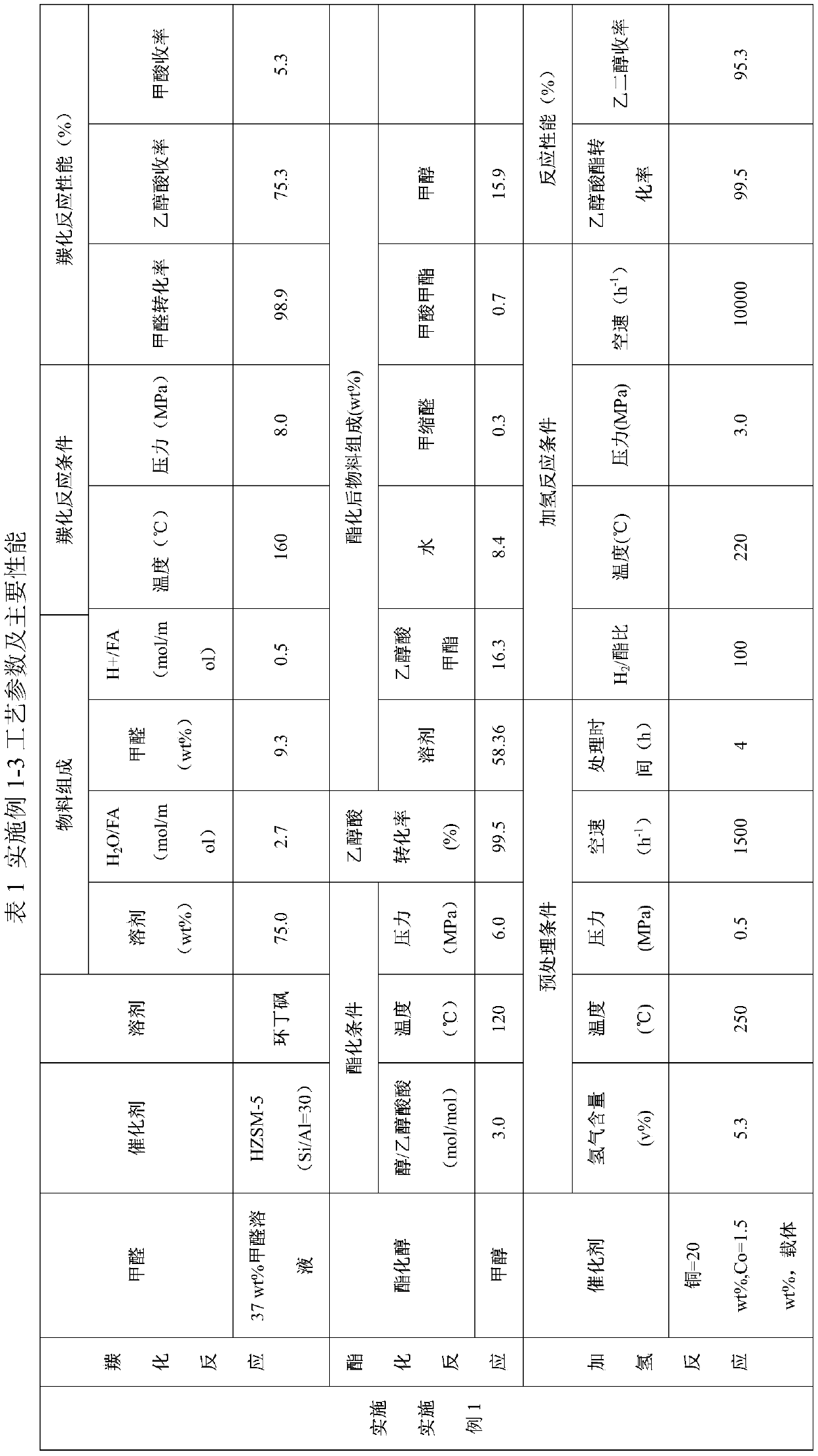
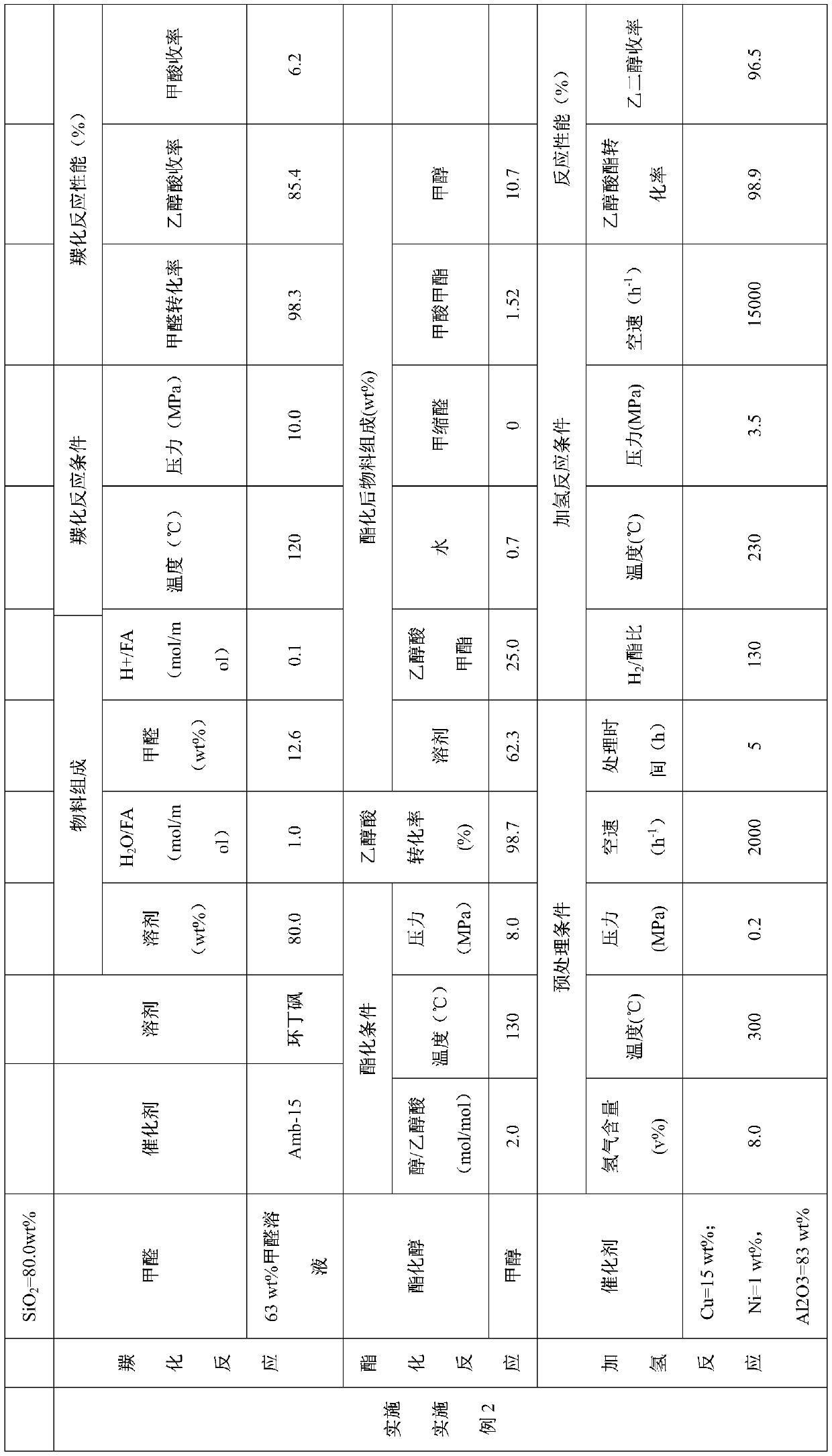
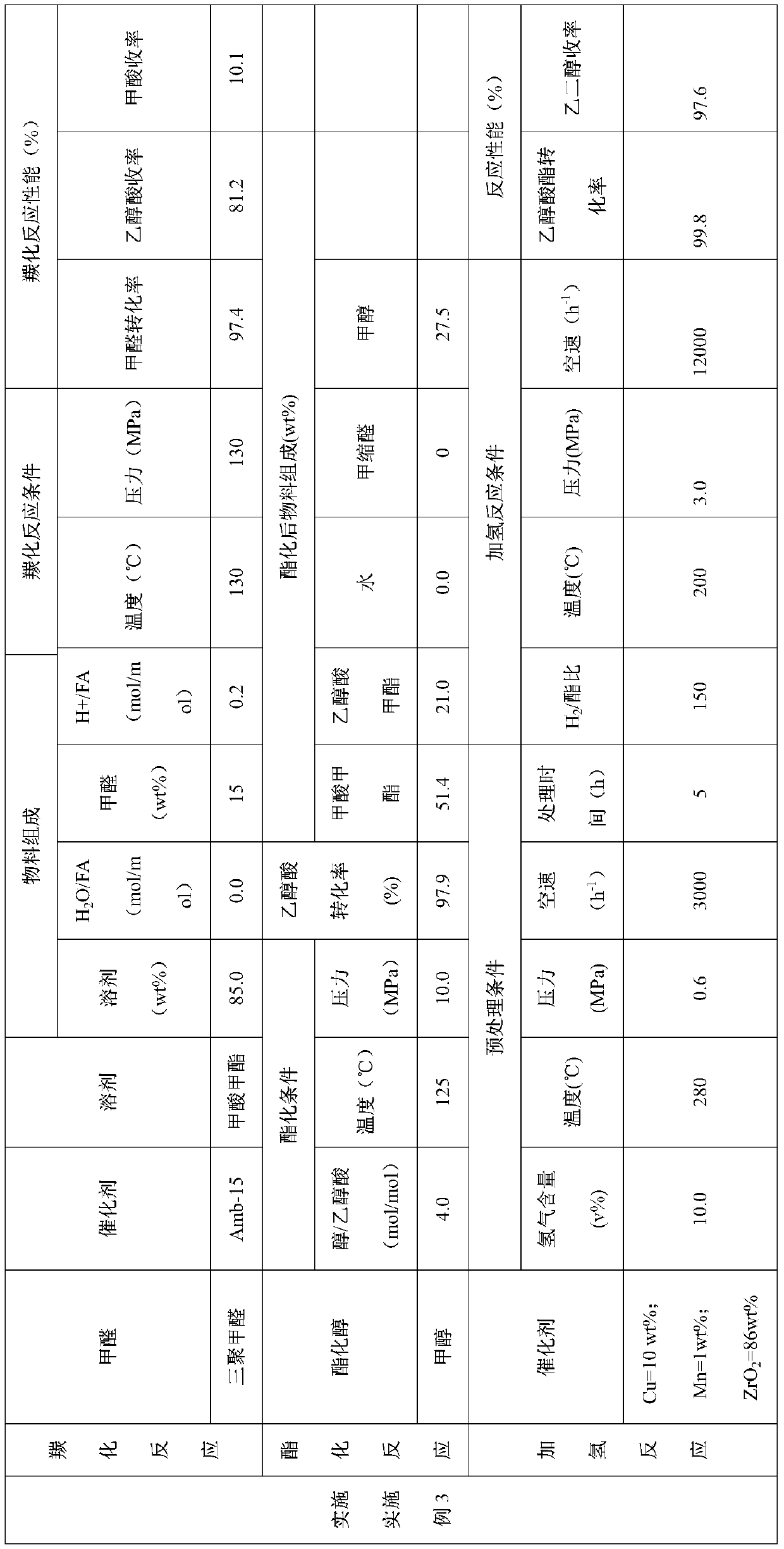
[0051] 37.0wt% formaldehyde, reaction solvent sulfolane and CO produced from methanol cracking enter the carbonylation esterification reactor filled with HZSM-5 (Si / Al molar ratio is 30) molecular sieves for carbonylation reaction. The mixed raw material is composed of: the mass content of sulfolane in the liquid phase raw material is 75.0wt%, the mass content of formaldehyde in the liquid phase raw material is 9.3wt%, H 2 The O / formaldehyde molar ratio was 2.7. The carbonylation reaction temperature is 160°C and the pressure is 8.0MPa. The performance of the carbonylation reaction is shown in Table 1.
[0052] Methanol enters the reactor from the middle of the carbonylation esterification reactor to carry out the esterification reaction. The esterification reaction conditions are as follows: the molar ratio of methanol and glycolic acid is 3.0, the reaction temperature is 120° C., and the reaction pressure is 6.0 MPa. The performance of the esterification reaction is shown...
Embodiment 2
[0056] The technological process of embodiment 2 and embodiment 3 is consistent with embodiment 1, and some parameters of modification are shown in table 1.
Embodiment 4
[0058] Paraformaldehyde, water, trifluoromethanesulfonic acid, sulfolane and CO from methanol cracking enter the carbonylation esterification reactor. The mixed raw material is composed of: the mass content of sulfolane in the liquid phase raw material is 75.0wt%, the mass content of paraformaldehyde in the liquid phase raw material is 10.0wt%, H 2 The molar ratio of O and formaldehyde is 1.0, and the mass content of trifluoromethanesulfonic acid in the liquid phase raw material is 2.0 wt%. The carbonylation reaction temperature is 120°C and the pressure is 8.0MPa. The carbonylation reaction performance is shown in Table 2.
[0059] Methanol enters the reactor from the middle of the carbonylation esterification reactor to carry out the esterification reaction. The esterification reaction conditions are as follows: the methanol / glycolic acid molar ratio is 2.0, the reaction temperature is 120° C., and the reaction pressure is 8.0 MPa. The esterification reaction performance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com