Synthesis method of bambuterol hydrochloride impurity B
A technology of bambuterol hydrochloride and a synthesis method, applied in the field of organic chemical synthesis, can solve problems such as less bambuterol hydrochloride impurity B and the like, and achieve the effects of easy availability of raw materials, short steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
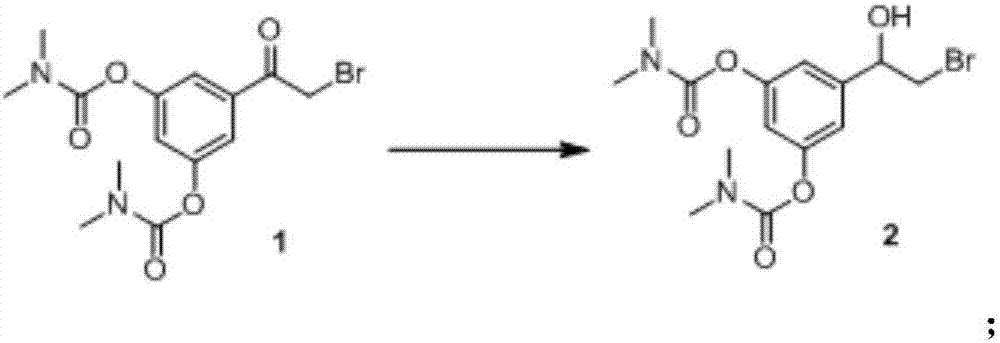
[0052] 5.0 grams of formula 1 compound [5-(2-bromoacetyl)-1,3-phenylene-bis(N,N-dimethylcarbamate)] was dissolved in 150 milliliters of anhydrous methanol, and the system Temperature down to 0-5°C, 1.0 g NaBH 4Add to the above solution several times. After the addition, the system is raised to normal temperature (25-30°C) and stirred for 2 hours. After concentrating to dryness under reduced pressure, add 50 ml of water to dissolve, extract 3 times with ethyl acetate (50 ml*3), wash 2 times with water (100 ml*2), wash 2 times with saturated brine (100 ml*2), no Dry over sodium sulfate for 2 hours, filter, wash with an appropriate amount of ethyl acetate, and concentrate under reduced pressure to obtain the compound of formula 2 [5-(2-bromo-1-hydroxyethyl)-1,3-phenylene-bis(N , N-dimethylcarbamate)] 4.1 g, yield 81.6%, purity 95%.
[0053] After confirmation and characterization using existing technologies, the obtained compound of formula 2 is 5-(2-bromo-1-hydroxyethyl)-1,3-p...
Embodiment 2-1
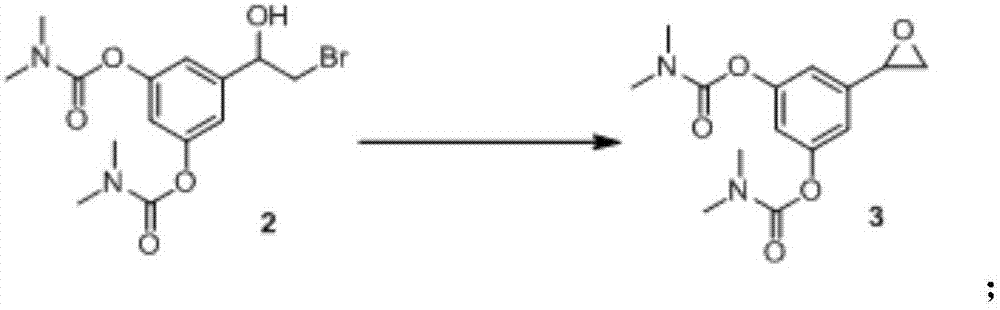
[0059] 4.1 grams of formula 2 compound [5-(2-bromo-1-hydroxyethyl)-1,3-phenylene-bis(N,N-dimethylcarbamate)] was dissolved in 120 milliliters of absolute ethanol , add 2 mol / L sodium hydroxide aqueous solution to the above reaction system, stir at room temperature (25-30° C.) for 15-20 minutes, and concentrate absolute ethanol under reduced pressure. After concentration to dryness, 50 ml of ethyl acetate and 40 ml of purified water were separated and extracted, and the aqueous phase was extracted once with 50 ml of ethyl acetate. The organic phases were combined, washed with water (100 ml), washed with saturated brine (100 ml), dried over anhydrous sodium sulfate for 2 hours, filtered, washed with an appropriate amount of ethyl acetate, and concentrated under reduced pressure to obtain the compound of formula 3 [5-(2-ring Oxyethyl)-1,3-phenylene-bis(N,N-dimethylcarbamate)] 2.9 g, yield: 90.2%, purity: 93%.
[0060] After confirmation and characterization using existing techno...
Embodiment 3
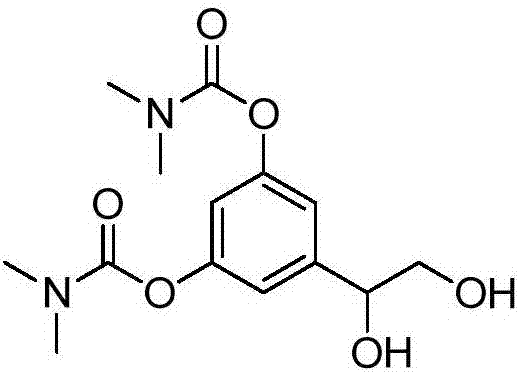
[0064] Example 3 Synthesis and preparation of bambuterol hydrochloride impurity B[5-(1,2-dihydroxyethyl)-1,3-phenylene-bis(N,N-dimethylcarbamate)]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com